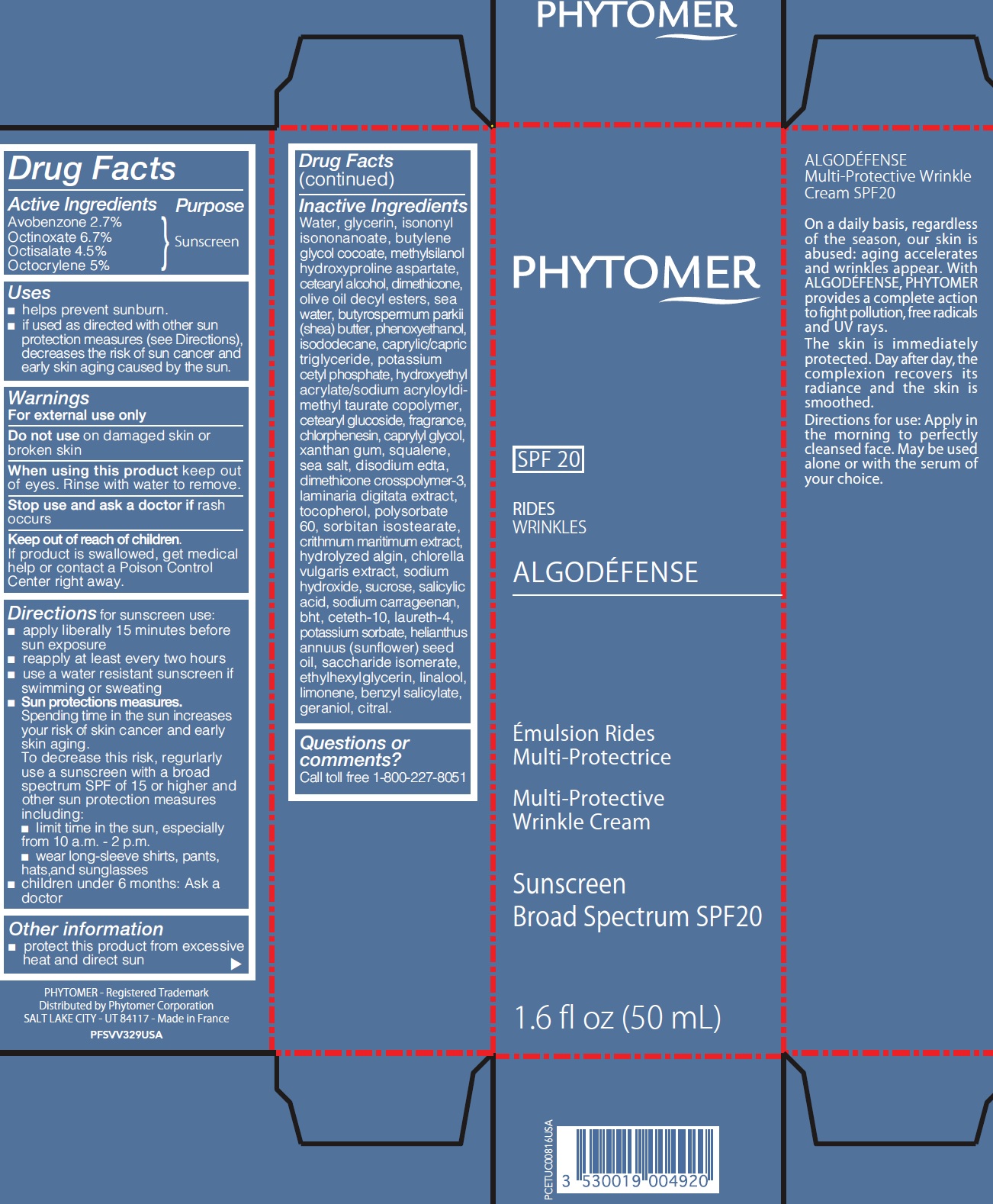
ALGODEFENSE RIDES MULTI PROTECTRICE MULTI-PROTECTIVE WRINKLE CREAM SUNSCREEN BROAD SPECTRUM SPF20- avobenzone, octinoxate, octisalate, octocrylene cream
Codif International
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Avobenzone 2.7%
Octinoxate 6.7%
Octisalate 4.5%
Octocrylene 5%
Uses
- helps prevent sunburn.
- if used as directed with other sun protection measures (see Directions), decreases the risk of sun cancer and early skin aging caused by the sun.
Warnings
For external use only
Do not use
on damaged skin or broken skin
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor
if rash occurs
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun protections measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regurlarly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Other information
- protect this product from excessive
heat and direct sun
Inactive Ingredients
Water, glycerin, isononyl isononanoate, butylene glycol cocoate, methylsilanol hydroxyproline aspartate, cetearyl alcohol, dimethicone, olive oil decyl esters, sea water, butyrospermum parkii (shea) butter, phenoxyethanol, isododecane, caprylic/capric triglyceride, potassium cetyl phosphate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, cetearyl glucoside, fragrance, chlorphenesin, caprylyl glycol, xanthan gum, squalene, sea salt, disodium edta, dimethicone crosspolymer-3, laminaria digitata extract, tocopherol, polysorbate 60, sorbitan isostearate, crithmum maritimum extract, hydrolyzed algin, chlorella vulgaris extract, sodium hydroxide, sucrose, salicylic acid, sodium carrageenan, bht, ceteth-10, laureth-4, potassium sorbate, helianthus annuus (sunflower) seed oil, saccharide isomerate, ethylhexylglycerin, linalool, limonene, benzyl salicylate, geraniol, citral.
Questions or comments?
Call toll free 1-800-227-8051
Package Labeling: