Label: XYZAL ALLERGY 24HR- levocetirizine dihydrochloride tablet
-
NDC Code(s):
41167-3510-0,
41167-3510-1,
41167-3510-2,
41167-3510-3, view more41167-3510-4, 41167-3510-5
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 16, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
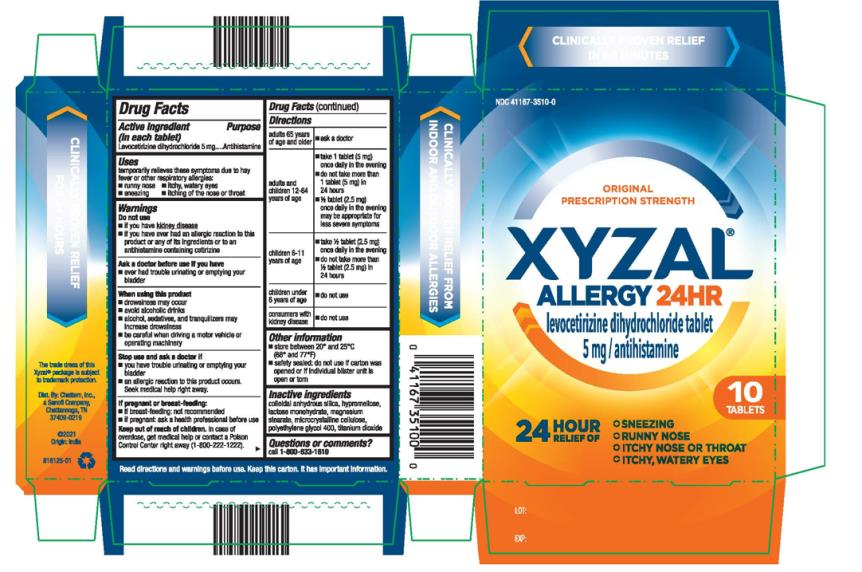
- Active ingredient
- Purpose
- Uses
-
Warnings
Do not use
- if you have kidney disease
- if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing cetirizine
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- you have trouble urinating or emptying your bladder
- an allergic reaction to this product occurs. Seek medical help right away.
- if you have kidney disease
-
Directions
adults 65 years of age and older ■ ask a doctor adults and children 12-64 years of age ■ take 1 tablet (5 mg) once daily in the evening
■ do not take more than 1 tablet (5 mg) in 24 hours
■ ½ tablet (2.5 mg) once daily in the evening may be appropriate for less severe symptomschildren 6-11 years of age ■ take ½ tablet (2.5 mg) once daily in the evening
■ do not take more than ½ tablet (2.5 mg) in 24 hourschildren under 6 years of age ■ do not use consumers with kidney disease ■ do not use (Note: Age ranges are bolded in the draft container labeling for tablet bottle)
-
Other information
- store between 20° and 25°C (68° and 77°F)
- (if blister) safety sealed: do not use if carton was opened or if individual blister unit is open or torn
(if bottled) safety sealed: do not use if carton was opened or if printed foil inner seal on bottle is torn or missing
(if stretch card) safety sealed: do not use if sealed package was torn or opened, or if printed foil inner seal on bottle is torn or missing
- store between 20° and 25°C (68° and 77°F)
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
XYZAL ALLERGY 24HR
levocetirizine dihydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-3510 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOCETIRIZINE DIHYDROCHLORIDE (UNII: SOD6A38AGA) (LEVOCETIRIZINE - UNII:6U5EA9RT2O) LEVOCETIRIZINE DIHYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score 2 pieces Shape OVAL Size 8mm Flavor Imprint Code X;X Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-3510-0 1 in 1 CARTON 03/10/2017 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:41167-3510-1 1 in 1 CARTON 03/10/2017 2 35 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:41167-3510-2 1 in 1 CARTON 03/10/2017 3 55 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:41167-3510-3 1 in 1 CARTON 03/10/2017 4 80 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:41167-3510-4 2 in 1 CARTON 03/10/2017 5 55 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:41167-3510-5 1 in 1 CARTON 01/04/2024 6 45 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209089 03/10/2017 Labeler - Chattem, Inc. (003336013)