Label: DEXTROSE- dextrose monohydrate injection, solution
-
NDC Code(s):
0990-7922-02,
0990-7922-03,
0990-7922-09,
0990-7922-53, view more0990-7922-55, 0990-7922-61, 0990-7923-13, 0990-7923-20, 0990-7923-23, 0990-7923-36, 0990-7923-37, 0990-7930-02, 0990-7930-03, 0990-7930-09
- Packager: ICU Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 18, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DEXTROSE INJECTION safely and effectively. See full prescribing information for DEXTROSE INJECTION.
DEXTROSE Injection, for intravenous use
Initial U.S. Approval: 1940RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Dextrose Injection is an intravenous solution indicated for parenteral replenishment of fluid and minimal carbohydrate calories as required by the clinical condition of the patient. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
WARNINGS AND PRECAUTIONS
- Hyperglycemia or Hyperosmolar Hyperglycemic State: Monitor blood glucose and administer insulin as needed. (5.1)
- Hypersensitivity Reactions: Monitor for signs and symptoms and discontinue infusion if reaction occurs. (5.2)
- Vein Damage and Thrombosis: Consider central vein when administering more than 5% dextrose or with an osmolarity of at least 900 mOsm/L or when there is peripheral vein irritation, phlebitis, and/or associated pain. (5.3)
- Hyponatremia: Avoid in patients with or at risk for hyponatremia. If use cannot be avoided, monitor serum sodium concentrations. (5.4)
- Electrolyte Imbalance and Fluid Overload: Avoid in patients with or at risk for fluid and/or solute overloading. If use cannot be avoided, monitor daily fluid balance, electrolyte concentrations, and acid-base balance, as needed and especially during prolonged use. (5.5)
- Refeeding Syndrome: Monitor severely undernourished patients and slowly increase nutrient intake. (5.6)
ADVERSE REACTIONS
The most common adverse reactions are hyperglycemia, hypersensitivity reactions, hyponatremia, infection both systemic and at the injection site, vein thrombosis or phlebitis, and electrolyte imbalance. (6)
To report SUSPECTED ADVERSE REACTIONS, contact ICU Medical, Inc. at 1-800-441-4100 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Additives may be incompatible. When introducing additives, use aseptic technique, mix thoroughly and do not store. (7)
USE IN SPECIFIC POPULATIONS
Pediatric Use: Increased risk of hypoglycemia/hyperglycemia; monitor serum glucose concentrations. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
2.2 Dosing Information
2.3 Discontinuation of Dextrose Injection
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hyperglycemia and Hyperosmolar Hyperglycemic State
5.2 Hypersensitivity Reactions
5.3 Vein Damage and Thrombosis
5.4 Hyponatremia
5.5 Electrolyte Imbalance and Fluid Overload
5.6 Refeeding Syndrome
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer unless solution is clear and container is undamaged. Discard unused portion.
To Open:
Tear outer wrap at notch and remove solution container. If supplemental medication is desired, follow directions below before preparing for administration.
To Add Medication:
- Prepare additive port.
- Using aseptic technique and an additive delivery needle of appropriate length, puncture resealable additive port at target area, inner diaphragm and inject. Withdraw needle after injecting medication.
- The additive port may be protected by covering with an additive cap.
- Mix container contents thoroughly.
Preparation for Administration
(Use aseptic technique)
- Close flow control clamp of administration set.
- Remove cover from outlet port at bottom of container.
- Insert piercing pin of administration set into port with a twisting motion until the set is firmly seated. NOTE: When using a vented administration set, replace bacterial retentive air filter with piercing pin cover. Insert piercing pin with twisting motion until shoulder of air filter housing rests against the outlet port flange.
- Suspend container from hanger.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- Attach venipuncture device to set.
- Open clamp to expel air from set and venipuncture device. Close clamp.
- Perform venipuncture.
- Regulate rate of administration with flow control clamp.
2.2 Dosing Information
The choice of dextrose concentration, rate and volume depends on the age, weight, clinical and metabolic conditions of the patient and concomitant therapy. Electrolyte supplementation may be indicated according to the clinical needs of the patient.
The administration rate should be governed, especially for premature infants with low birth weight, during the first few days of therapy, by the patient's tolerance to dextrose.
Increase the infusion rate gradually as indicated by frequent monitoring of blood glucose concentrations [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
-
3 DOSAGE FORMS AND STRENGTHS
Dextrose Injection, USP is a clear, sterile, nonpyrogenic solution of dextrose supplied in single-dose flexible plastic containers:
- 5% (0.05 grams/mL): 5 grams of dextrose hydrous per 100 mL in flexible containers: 25 mL, 50 mL, 100 mL, 150 mL, 250 mL, 500 mL, and 1000 mL
- 10% (0.1 grams/mL): 10 grams of dextrose hydrous per 100 mL in flexible containers: 250 mL, 500 mL, and 1000 mL
-
4 CONTRAINDICATIONS
The use of Dextrose Injection is contraindicated in patients with:
- Clinically significant hyperglycemia [see Warnings and Precautions (5.1)].
- Known hypersensitivity to dextrose [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hyperglycemia and Hyperosmolar Hyperglycemic State
The use of dextrose infusions in patients with impaired glucose tolerance may worsen hyperglycemia. Administration of dextrose at a rate exceeding the patient's utilization rate may lead to hyperglycemia, coma, and death.
Hyperglycemia is associated with an increase in serum osmolality, resulting in osmotic diuresis, dehydration and electrolyte losses [see Warnings and Precautions (5.5)]. Patients with underlying CNS disease and renal impairment who receive dextrose infusions, may be at greater risk of developing hyperosmolar hyperglycemic state.
Monitor blood glucose levels and treat hyperglycemia to maintain levels within normal limits while administering Dextrose Injection. Insulin may be administered or adjusted to maintain optimal blood glucose levels during Dextrose Injection administration.
5.2 Hypersensitivity Reactions
Hypersensitivity and infusion reactions, including anaphylaxis, have been reported with Dextrose Injection [see Adverse Reactions (6)]. Stop infusion immediately and treat patient accordingly if signs or symptoms of a hypersensitivity reaction develop. Appropriate therapeutic countermeasures must be instituted as clinically indicated.
5.3 Vein Damage and Thrombosis
Peripheral administration of 5% Dextrose Injection is generally acceptable, however, consider central vein when administering more than 5% dextrose or with an osmolarity of ≥ at least 900 mOsm/L or when there is peripheral vein irritation, phlebitis, and/or associated pain. The infusion of hypertonic solutions into a peripheral vein may result in vein irritation, vein damage, and/or thrombosis. The primary complication of peripheral access is venous thrombophlebitis, which manifests as pain, erythema, tenderness or a palpable cord. Remove the catheter as soon as possible, if thrombophlebitis develops.
5.4 Hyponatremia
10% Dextrose Injection is a hypertonic solution [see Description (11)]. In the body, however, glucose containing fluids can become extremely physiologically hypotonic due to rapid glucose metabolization. Monitoring of serum sodium is particularly important for hypotonic fluids.
Depending on the tonicity of the solution, the volume and rate of infusion, and depending on a patient's underlying clinical condition and capability to metabolize glucose, intravenous administration of glucose can cause electrolyte disturbances, most importantly hypo-or hyperosmotic hyponatremia. Monitor serum sodium to minimize the risk of hyponatremia.
The risk for hyponatremia is increased in pediatric patients, elderly patients, postoperative patients, those with psychogenic polydipsia, and in patients treated with medications that increase the risk of hyponatremia (such as diuretics, certain antiepileptic and psychotropic medications). Close clinical monitoring may be warranted.
Acute hyponatremia can lead to acute hyponatremic encephalopathy characterized by headache, nausea, seizures, lethargy and vomiting. Patients with brain edema are at particular risk of severe, irreversible and life-threatening brain injury. Patients at increased risk for developing complications of hyponatremia, such as hyponatremic encephalopathy include pediatric patients; women, in particular, premenopausal women; patients with hypoxemia; and in patients with underlying central nervous system disease [see Use in Specific Populations (8.4, 8.5)].
Avoid Dextrose Injection in patients with or at risk for hyponatremia. If use cannot be avoided, monitor serum sodium concentrations.
Rapid correction of hyponatremia is potentially dangerous with risk of serious neurologic complications. Brain adaptations reducing risk of cerebral edema make the brain vulnerable to injury when chronic hyponatremia is too rapidly corrected, which is known as osmotic demyelination syndrome (ODS). To avoid complications, monitor serum sodium and chloride concentrations, fluid status, acid-base balance, and signs of neurologic complications.
High volume infusion must be used with close monitoring in patients with cardiac or pulmonary failure, and in patients with non-osmotic vasopressin release (including SIADH), due to the risk of hospital-acquired hyponatremia.
5.5 Electrolyte Imbalance and Fluid Overload
Electrolyte deficits, particularly in serum potassium and phosphate, may occur during prolonged use of concentrated dextrose solutions.
Depending on the volume and rate of infusion, the patient's underlying clinical condition and capability to metabolize dextrose, intravenous administration of Dextrose Injection can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, (including hypoosmotic hyponatremia), overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentrations in the administered solution. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations in the solution.
Avoid Dextrose Injection in patients with or at risk for fluid and/or solute overloading. If use cannot be avoided, monitor fluid balance, blood electrolyte levels, concentration of glucose, acid-base balance, correct fluid and electrolyte imbalances during prolonged parenteral therapy or whenever the condition of the patient or the rate of administration warrants such evaluation and acid-base balance as needed and especially during prolonged use. Additional monitoring is recommended for patients with water and electrolyte disturbances that could be aggravated by increased glucose, insulin administration and/or free water load. Patients at increased risk for developing hyponatremic encephalopathy include pediatric patients; elderly patients, women, in particular premenopausal women; patients with hypoxemia; and patients with underlying CNS disease [see Use in Specific Populations (8.4, 8.5)].
5.6 Refeeding Syndrome
Refeeding severely undernourished patients may result in refeeding syndrome, characterized by the intracellular shift of potassium, phosphorus, and magnesium as the patient becomes anabolic. Thiamine deficiency and fluid retention may also develop. To prevent these complications, monitor severely undernourished patients and slowly increase nutrient intake.
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of Dextrose Injection were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hyperglycemia and hyperosmolar hyperglycemic state [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions: anaphylaxis, pruritis, bronchospasm, cyanosis, angioedema, hypotension, pyrexia, chills, and rash [see Warnings and Precautions (5.2)]
- Infusion Site Reactions: infusion site phlebitis, infusion site erythema, vein damage and thrombosis, and infusion site thrombophlebitis (10% dextrose only) [see Warnings and Precautions (5.3)]
- Hyponatremia and hyponatremic encephalopathy [see Warnings and Precautions (5.4)]
- Electrolyte Imbalance and Fluid Overload [see Warnings and Precautions (5.5)]
- Refeeding syndrome [see Warnings and Precautions (5.6)]
- Pulmonary vascular precipitates (10% dextrose only)
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Appropriate administration of Dextrose Injection during pregnancy is not expected to cause adverse developmental outcomes, including congenital malformations. Animal reproduction studies have not been conducted with injectable dextrose solutions.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of dextrose in human milk, the effects on a breastfed infant, or the effects on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of Dextrose Injection to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Dextrose Injection and any potential adverse effects on the breastfed infant from Dextrose Injection or from the underlying maternal condition.
8.4 Pediatric Use
The safety profile of Dextrose Injection in pediatric patients is similar to adults.
Neonates, especially premature infants with low birth weight, are at increased risk of developing hypo-or hyperglycemia and therefore need close monitoring during treatment with intravenous glucose infusions to ensure adequate glycemic control in order to avoid potential long-term adverse effects.
Closely monitor plasma electrolyte concentrations in pediatric patients who may have impaired ability to regulate fluids and electrolytes. In very low birth weight infants, excessive or rapid administration of Dextrose Injection may result in increased serum osmolality and risk of intracerebral hemorrhage.
Children (including neonates and older children) are at increased risk of developing hyponatremia as well as for developing hyponatremic encephalopathy [see Warnings and Precautions (5.4)].
8.5 Geriatric Use
Clinical studies of Dextrose Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Elderly patients are at increased risk of developing hyponatremia as well as for developing hyponatremic encephalopathy [see Warnings and Precautions (5.4)]. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Dextrose is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
10 OVERDOSAGE
An increased infusion rate of Dextrose Injection or administration of dextrose solutions can cause hyperglycemia, hyperosmolality, and adverse effects on water and electrolyte balance [see Warnings and Precautions (5.1, 5.5)].
Severe hyperglycemia and severe dilutional hyponatremia, and their complications, can be fatal. Discontinue infusion, reduce dose and institute appropriate corrective measures such as administration of exogenous insulin.
Discontinue infusion and institute appropriate corrective measures in the event of overhydration or solute overload during therapy, with particular attention to CNS, respiratory and cardiovascular systems.
If over-exposure occurs, contact the Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage.
-
11 DESCRIPTION
Dextrose Injection, USP solutions are sterile and nonpyrogenic. They are parenteral solutions containing various concentrations of dextrose in water for injection intended for intravenous administration.
Each 100 mL of 5% Dextrose Injection, USP, contains dextrose, hydrous 5 g in water for injection. The caloric value is 170 kcal/L. The osmolarity is 252 mOsmol/L (calc.), which is slightly hypotonic.
Each 100 mL of 10% Dextrose Injection, USP, contains dextrose, hydrous 10 g in water for injection. The caloric value is 340 kcal/L. The osmolarity is 505 mOsmol/L (calc.), which is hypertonic.
The pH for both concentrations is 4.3 (3.2 to 6.5).
The solutions contain no bacteriostat, antimicrobial agent or added buffer and each is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
The solutions are parenteral fluid and nutrient replenishers.
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 ∙ H2O), a hexose sugar freely soluble in water. It has the following structural formula:
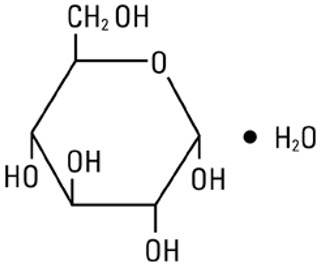
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
When administered intravenously, these solutions provide a source of water and carbohydrate.
12.2 Pharmacodynamics
Isotonic and hypertonic concentrations of dextrose are suitable for parenteral maintenance of water requirements when salt is not needed or should be avoided.
Solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirements range from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Dextrose Injection, USP is a clear, sterile, nonpyrogenic solution of dextrose supplied in single-dose flexible plastic containers as shown in the accompanying Table.
NDC No. Product Container size
(mL)0409-7922-61* 5% Dextrose Injection, USP 150 0990-7922-61* 5% Dextrose Injection, USP 150 0409-7922-53† 5% Dextrose Injection, USP 250 0990-7922-53† 5% Dextrose Injection, USP 250 0409-7922-02* 5% Dextrose Injection, USP 250 0990-7922-02* 5% Dextrose Injection, USP 250 0409-7922-03† 5% Dextrose Injection, USP 500 0990-7922-03*,† 5% Dextrose Injection, USP 500 0409-7922-55* 5% Dextrose Injection, USP 500 0990-7922-55*,† 5% Dextrose Injection, USP 500 0409-7922-09*,† 5% Dextrose Injection, USP 1000 0990-7922-09*,† 5% Dextrose Injection, USP 1000 0409-7923-20* 5% Dextrose Injection, USP 25 0990-7923-20* 5% Dextrose Injection, USP 25 0409-7923-36* 5% Dextrose Injection, USP 50 0990-7923-36* 5% Dextrose Injection, USP 50 0409-7923-13* 5% Dextrose Injection, USP 50 0990-7923-13* 5% Dextrose Injection, USP 50 0409-7923-23* 5% Dextrose Injection, USP 100 0990-7923-23* 5% Dextrose Injection, USP 100 0409-7923-37* 5% Dextrose Injection, USP 100 0990-7923-37* 5% Dextrose Injection, USP 100 0409-7930-02* 10% Dextrose Injection, USP 250 0990-7930-02* 10% Dextrose Injection, USP 250 0409-7930-03† 10% Dextrose Injection, USP 500 0990-7930-03*,† 10% Dextrose Injection, USP 500 0409-7930-09† 10% Dextrose Injection, USP 1000 0990-7930-09*,† 10% Dextrose Injection, USP 1000 ICU Medical is transitioning NDC codes from "0409" to "0990" labeler code. Both NDC codes are expected to be in the market for a period of time.
-
17 PATIENT COUNSELING INFORMATION
Inform patients, caregivers, or home healthcare providers of the following risks of Dextrose Injection:
- Hyperglycemia and hyperosmolar hyperglycemic state [see Warnings and Precautions (5.1)]
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
- Vein damage and thrombosis [see Warnings and Precautions (5.3)]
- Hyponatremia [see Warnings and Precautions (5.4)]
- Electrolyte Imbalance and Fluid Overload [see Warnings and Precautions (5.5)]
- Refeeding syndrome [see Warnings and Precautions (5.6)]
- SPL UNCLASSIFIED SECTION
-
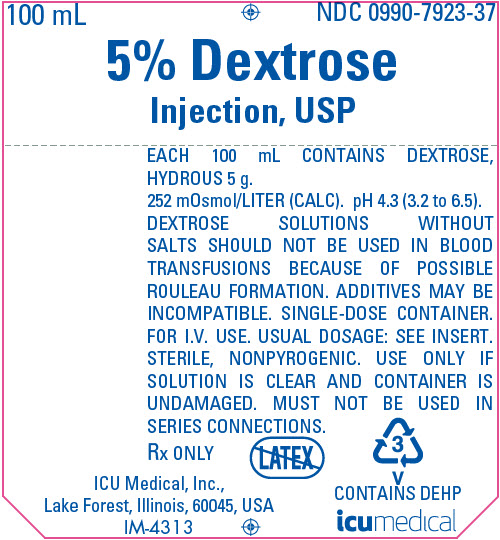
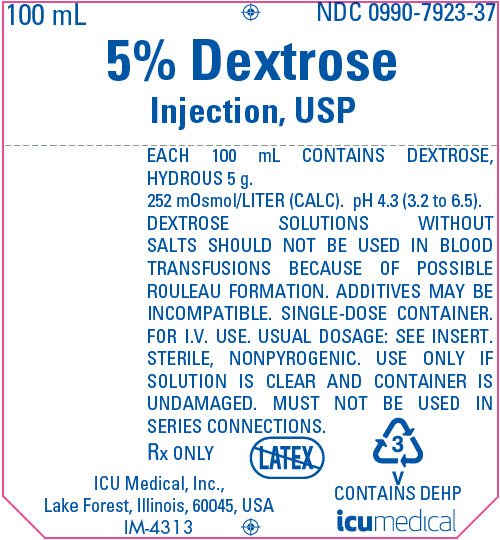
PRINCIPAL DISPLAY PANEL - 100 mL Bag Label
100 mL
NDC 0990-7923-375% Dextrose
Injection, USPEACH 100 mL CONTAINS DEXTROSE,
HYDROUS 5 g.
252 mOsmol/LITER (CALC). pH 4.3 (3.2 to 6.5).
DEXTROSE SOLUTIONS WITHOUT
SALTS SHOULD NOT BE USED IN BLOOD
TRANSFUSIONS BECAUSE OF POSSIBLE
ROULEAU FORMATION. ADDITIVES MAY BE
INCOMPATIBLE. SINGLE-DOSE CONTAINER.
FOR I.V. USE. USUAL DOSAGE: SEE INSERT.
STERILE, NONPYROGENIC. USE ONLY IF
SOLUTION IS CLEAR AND CONTAINER IS
UNDAMAGED. MUST NOT BE USED IN
SERIES CONNECTIONS.Rx ONLY
ICU Medical, Inc.,
Lake Forest, Illinois, 60045, USA
IM-43133
V
CONTAINS DEHPicumedical
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label
250 mL
NDC 0990-7922-535% DEXTROSE
Injection, USPEACH 100 mL CONTAINS DEXTROSE,
HYDROUS 5 g IN WATER FOR
INJECTION.
252 mOsmol/LITER (CALC.)
pH 4.3 (3.2 to 6.5)
DEXTROSE SOLUTIONS WITHOUT
SALTS SHOULD NOT BE USED IN
BLOOD TRANSFUSIONS BECAUSE OF
POSSIBLE ROULEAU FORMATION.
ADDITIVES MAY BE INCOMPATIBLE.
CONSULT WITH PHARMACIST, IF
AVAILABLE. WHEN INTRODUCING
ADDITIVES, USE ASEPTIC TECHNIQUE,
MIX THOROUGHLY AND DO NOT
STORE. SINGLE-DOSE CONTAINER.
FOR I.V. USE. USUAL DOSAGE: SEE
INSERT. STERILE, NONPYROGENIC.
USE ONLY IF SOLUTION IS CLEAR
AND CONTAINER IS UNDAMAGED.
MUST NOT BE USED IN SERIES
CONNECTIONS.Rx ONLY
3
V
CONTAINS DEHPIM-4428
Manufactured for ICU Medical, Inc.,
Lake Forest, Illinois, 60045, USAicumedical

-
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label
500 mL
NDC 0990-7930-0310% DEXTROSE
Injection, USPEACH 100 mL CONTAINS DEXTROSE, HYDROUS 10 g IN
WATER FOR INJECTION.
505 mOsmol/LITER (CALC.)
pH 4.3 (3.2 to 6.5)
DEXTROSE SOLUTIONS WITHOUT SALTS SHOULD
NOT BE USED IN BLOOD TRANSFUSIONS BECAUSE
OF POSSIBLE ROULEAU FORMATION. ADDITIVES MAY
BE INCOMPATIBLE. CONSULT WITH PHARMACIST,
IF AVAILABLE. WHEN INTRODUCING ADDITIVES, USE
ASEPTIC TECHNIQUE, MIX THOROUGHLY AND DO NOT
STORE. SINGLE-DOSE CONTAINER. FOR INTRAVENOUS
OR SUBCUTANEOUS USE. USUAL DOSAGE: SEE INSERT.
STERILE, NONPYROGENIC. USE ONLY IF SOLUTION IS
CLEAR AND CONTAINER IS UNDAMAGED. MUST NOT BE
USED IN SERIES CONNECTIONS.Rx ONLY
3
V
CONTAINS DEHPicumedical
ICU Medical, Inc., Lake Forest, Illinois, 60045, USA
IM-4454
-
INGREDIENTS AND APPEARANCE
DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0990-7923 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0990-7923-20 12 in 1 CASE 08/01/2019 1 4 in 1 POUCH 1 25 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:0990-7923-23 48 in 1 CASE 08/01/2019 2 1 in 1 POUCH 2 100 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC:0990-7923-36 20 in 1 CASE 07/15/2019 3 4 in 1 POUCH 3 50 mL in 1 BAG; Type 0: Not a Combination Product 4 NDC:0990-7923-37 20 in 1 CASE 07/15/2019 4 4 in 1 POUCH 4 100 mL in 1 BAG; Type 0: Not a Combination Product 5 NDC:0990-7923-13 48 in 1 CASE 08/01/2019 5 1 in 1 POUCH 5 50 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016367 07/15/2019 DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0990-7922 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0990-7922-02 24 in 1 CASE 08/01/2019 1 1 in 1 POUCH 1 250 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:0990-7922-03 24 in 1 CASE 08/01/2019 2 1 in 1 POUCH 2 500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC:0990-7922-09 12 in 1 CASE 09/01/2019 3 1 in 1 POUCH 3 1000 mL in 1 BAG; Type 0: Not a Combination Product 4 NDC:0990-7922-53 24 in 1 CASE 10/01/2019 4 1 in 1 POUCH 4 250 mL in 1 BAG; Type 0: Not a Combination Product 5 NDC:0990-7922-55 18 in 1 CASE 08/01/2019 5 1 in 1 POUCH 5 500 mL in 1 BAG; Type 0: Not a Combination Product 6 NDC:0990-7922-61 32 in 1 CASE 08/01/2019 6 1 in 1 POUCH 6 150 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016367 07/15/2019 DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0990-7930 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0990-7930-02 24 in 1 CASE 08/01/2019 1 1 in 1 POUCH 1 250 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:0990-7930-03 24 in 1 CASE 08/01/2019 2 1 in 1 POUCH 2 500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC:0990-7930-09 12 in 1 CASE 08/01/2019 3 1 in 1 POUCH 3 1000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018080 07/15/2019 Labeler - ICU Medical Inc. (118380146) Establishment Name Address ID/FEI Business Operations ICU MEDICAL INC. 117395980 ANALYSIS(0990-7922, 0990-7923, 0990-7930) , MANUFACTURE(0990-7922, 0990-7923, 0990-7930) , PACK(0990-7922, 0990-7923, 0990-7930) , LABEL(0990-7922, 0990-7923, 0990-7930)