Label: 4378 FIRST AID KIT kit
- NDC Code(s): 0498-0501-00, 0498-2110-01, 0498-4378-01
- Packager: Honeywell Safety Products USA, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- BZK Active ingredient
- BZK Purpose
- BZK Uses
-
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- BZK Directions
- BZK Other information
- BZK Inactiave ingredient
- BzK Questions
- Aypanal Active ingredient (in each tablet)
- Aypanal Purpose
- Aypanal Uses
-
Aypanal
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount.
- with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
Keep out of reach of children.
Keep out of reach of children.
Overdose warning: In case ofl overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
- Aypanal Directions
- Aypanal Other information
- Aypanal Inactive igredients
- Aypanal Questions or Comments
- FABC Active ingredient
- FABC Purpose
- FABC Uses
-
FABC
Warnings
For external use only
Do not use
- in or near the eyes
- if you are allergic to any of the ingredients
- lin large areas of the body, particularly over raw surfaces or blistered areas
- for more than 10 days
- FABC Directions
- FABC Inactive ingredients
- FABC Questions
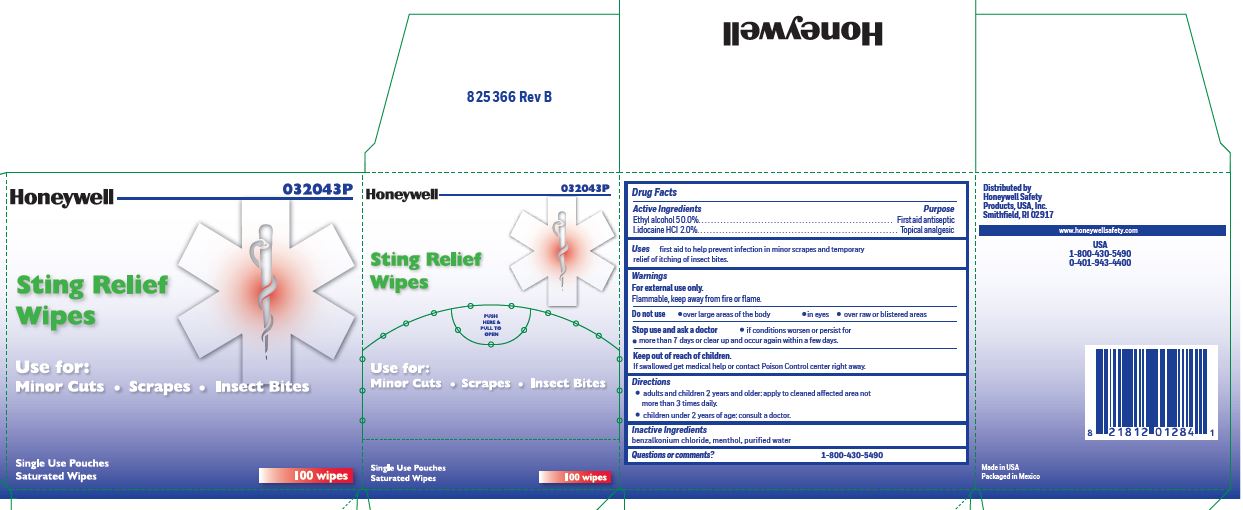
- Sting Relief Active ingredient (in eachwipe)
- Sting Relief Purpose
- Sting Rellief Uses
- Sting Relief Warnings
- Sting Relief Directions
- Sting Relief Inactive ingredients
- Sting Relief Questions or Comments?
-
4378
Z631580000 KIT CONTENTS
1 INSTANT COLD PACK 4" X 6"
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 FIRST AID GUIDE ASHI
1 ABD COMBINE PAD 5" X 9"
1 CPR FILTERSHIELD 77-100
1 FIRST AID BURN CREAM 0.9 GRM PKT 20
1 SCISSOR BDGE 4" RED PLS HDL
1 FANNY PACK RED FAK LOGO EMPTY
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
6 BZK ANTISEPTIC WIPE, BULK
1 1 PR LRG NITRILE GLVES ZIP BAG
1 1" X 3" PLASTIC BANDS 16/BAG
5 SAFETEC STING RELIEF WIPES BULK
1 TRI BNDG NON WOVEN 40"X40"X56"
3 GAUZE PADS 3"X3" 12PLY
2 HEAVY FLEX LARGE PATCH 2" X 3"
2 AYPANAL EXTRA BULK 2/PK
- BZK Principal Display Panel
- Aypanal Principal Display Panel
- FABC Principal Display Panel
- Sting Relief Principal Display Panel
- 4378 Kit Label Z631580000
-
INGREDIENTS AND APPEARANCE
4378 FIRST AID KIT
4378 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4378 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4378-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 5 POUCH 2 mL Part 2 6 PACKET 8.4 mL Part 3 2 PACKET 4 Part 4 20 PACKET 18 g Part 1 of 4 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC:0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/23/2017 Part 2 of 4 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC:0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/22/2017 Part 3 of 4 AYPANAL EX
acetaminophen tabletProduct Information Item Code (Source) NDC:0498-2110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor Imprint Code FR1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-2110-01 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/02/2017 Part 4 of 4 FIRST AID BURN
benzalkonium chloride, lidocaine hydrochloride creamProduct Information Item Code (Source) NDC:0498-0903 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) METHYLPARABEN (UNII: A2I8C7HI9T) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) LIGHT MINERAL OIL (UNII: N6K5787QVP) EDETATE DISODIUM (UNII: 7FLD91C86K) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) PROPYLPARABEN (UNII: Z8IX2SC1OH) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/20/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (118768815)