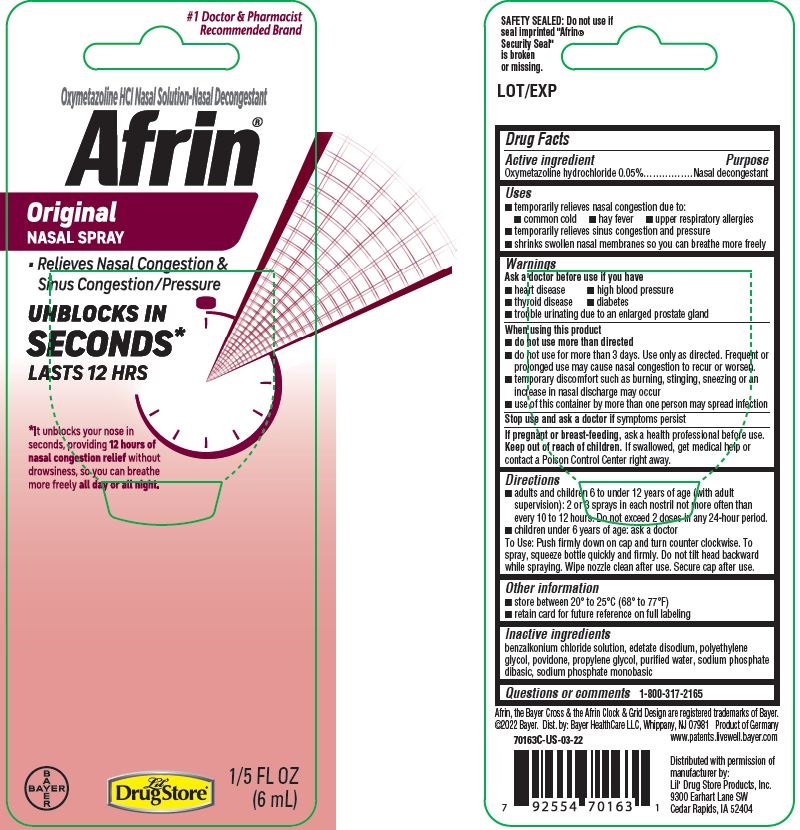
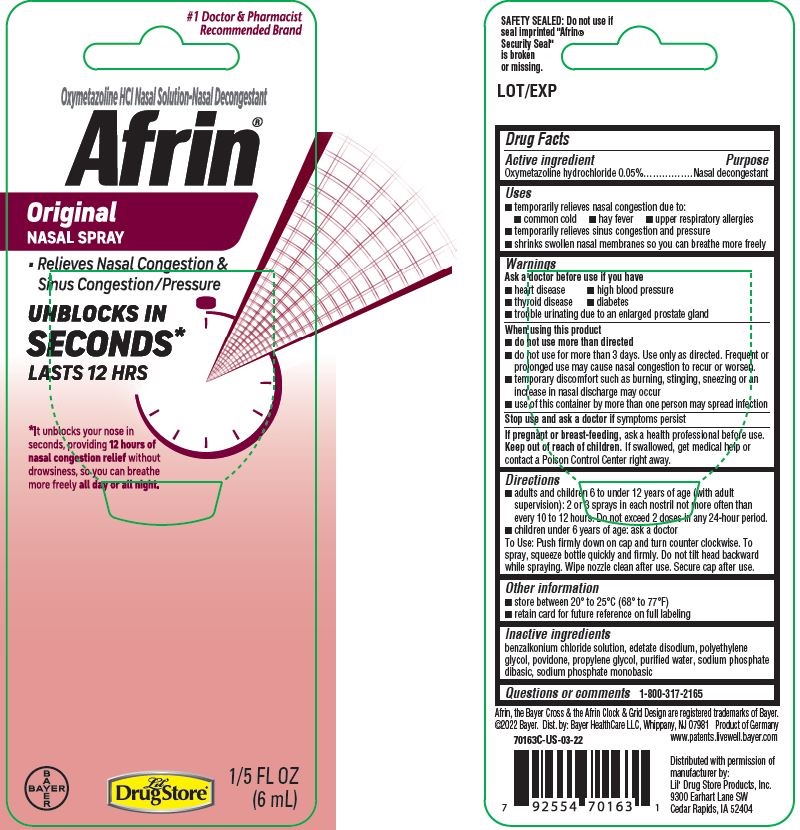
Label: AFRIN ORIGINAL- oxymetazoline hydrochloride spray
- NDC Code(s): 66715-5880-0, 66715-7016-3
- Packager: Lil' Drug Store Products, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 17, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection
-
Directions
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- children under 6 years of age: ask a doctor
To spray, squeeze bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use. Secure cap after use.
- Other information
- Inactive ingredients
- Questions or comments
-
PDP/PACKAGE - 6 mL Bottle Blister Pack, Lil' Drug Store
#1 Doctor & Pharmacist
Recommended BrandOxymetazoline HCl Nasal Solution-Nasal Decongestant
Afrin ® Original
NASAL SPRAY
- Relieves Nasal Congestion &
Sinus Congestion/Pressure
UNBLOCKS IN
SECONDS*
LASTS 12 HRS
*It unblocks your nose in
seconds, providing 12 hours of
nasal congestion relief without
drowsiness, so you can breathe
more freely all day or all night
[Bayer cross]
[Lil' Drug Store logo]
1/5 FL OZ
(6mL)
- PDP/Package - BASIX
-
INGREDIENTS AND APPEARANCE
AFRIN ORIGINAL
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-7016 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-7016-3 1 in 1 BLISTER PACK 11/09/2018 1 6 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/01/2008 AFRIN ORIGINAL
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-5880 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-5880-0 1 in 1 BLISTER PACK 02/13/2018 1 6 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 02/13/2018 Labeler - Lil' Drug Store Products, Inc. (093103646)