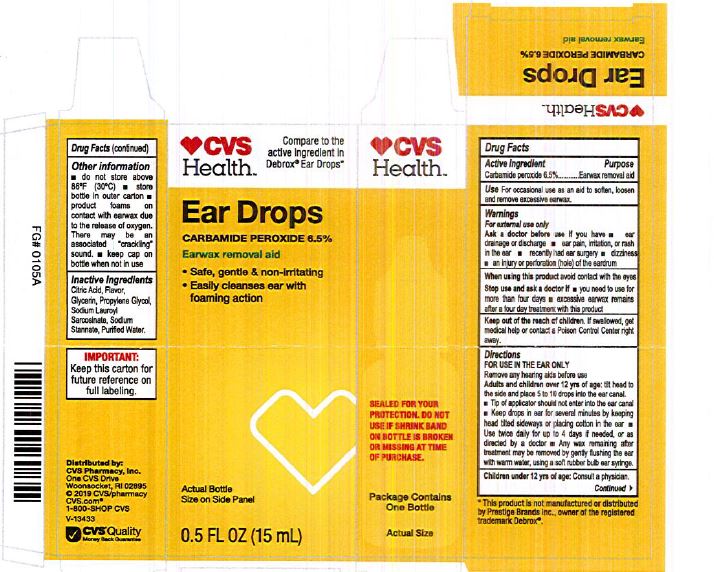
Label: EAR DROPS- carbamide peroxide 6.5% liquid
- NDC Code(s): 69842-097-45, 69842-097-90
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INACTIVE INGREDIENT
- PURPOSE
- DOSAGE & ADMINISTRATION
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EAR DROPS
carbamide peroxide 6.5% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-097 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 0.065 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM STANNATE (UNII: NJ7C1V83KG) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-097-90 1 in 1 CARTON 06/04/2019 1 15 mL in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:69842-097-45 1 in 1 CARTON 06/04/2019 2 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/04/2019 Labeler - CVS Pharmacy (062312574) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 manufacture(69842-097) , analysis(69842-097) , pack(69842-097) , label(69842-097)