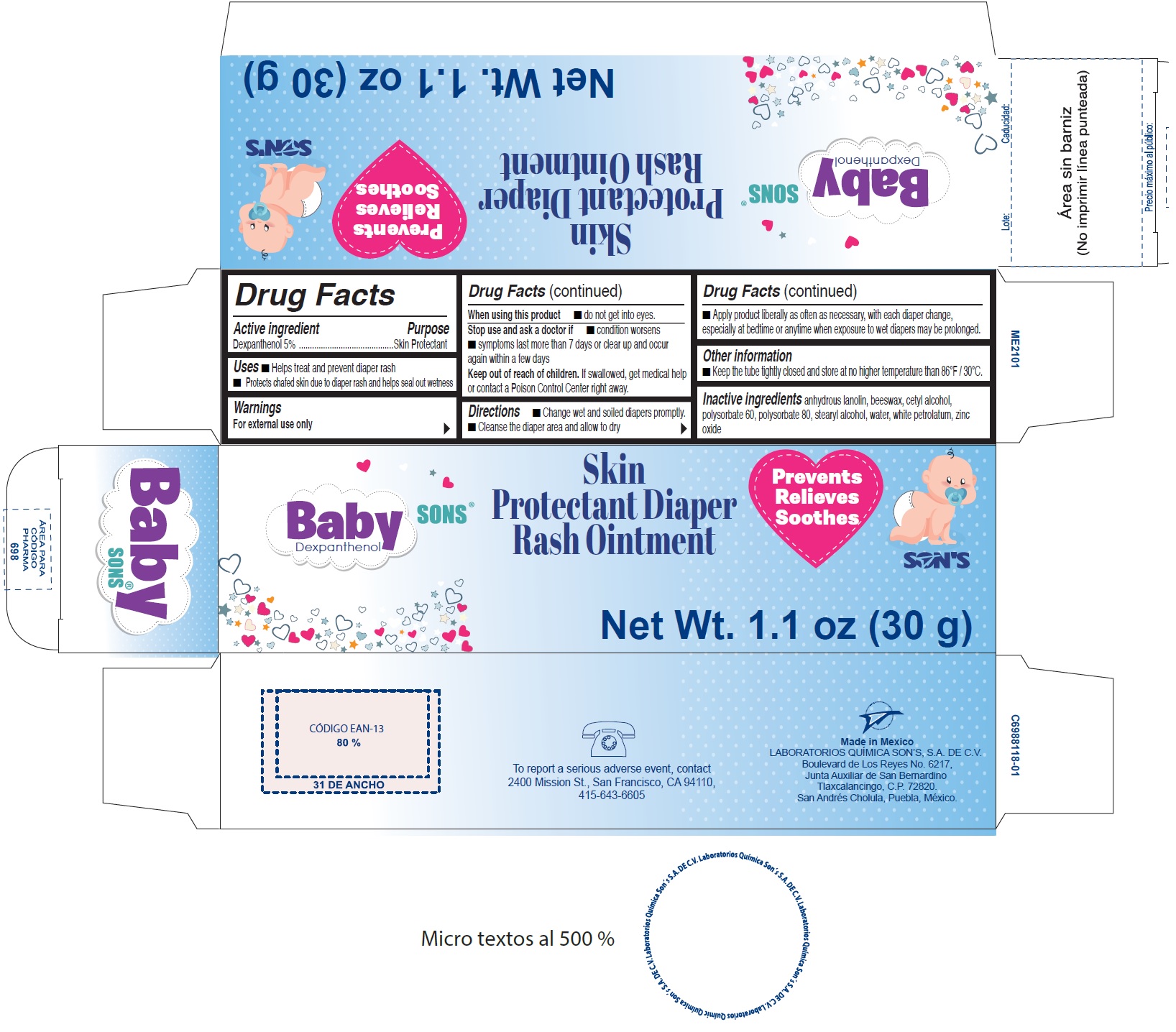
Label: BABYSONS- dexpanthenol ointment
- NDC Code(s): 73519-001-30
- Packager: Laboratorios Quimica Son's, S.A. de C.V
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 9, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BABYSONS
dexpanthenol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73519-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXPANTHENOL (UNII: 1O6C93RI7Z) (DEXPANTHENOL - UNII:1O6C93RI7Z) DEXPANTHENOL 50 mg in 1 g Inactive Ingredients Ingredient Name Strength LANOLIN (UNII: 7EV65EAW6H) YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ALCOHOL (UNII: 936JST6JCN) POLYSORBATE 60 (UNII: CAL22UVI4M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73519-001-30 1 in 1 BOX 06/15/2020 1 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/15/2020 Labeler - Laboratorios Quimica Son's, S.A. de C.V (815603378) Establishment Name Address ID/FEI Business Operations Laboratorios Quimica Son's, S.A. de C.V 815603378 manufacture(73519-001)