4359 FIRST AID KIT- 4359 first aid kit
Honeywell Safety Products USA, INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-4359: First Aid Kit (ammonia inh, EW, BZK wipes, Burn Jel, PVP wipes, PAWS- 68WP3-01)
Eyesaline
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyesaline
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyesaline
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyesaline
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
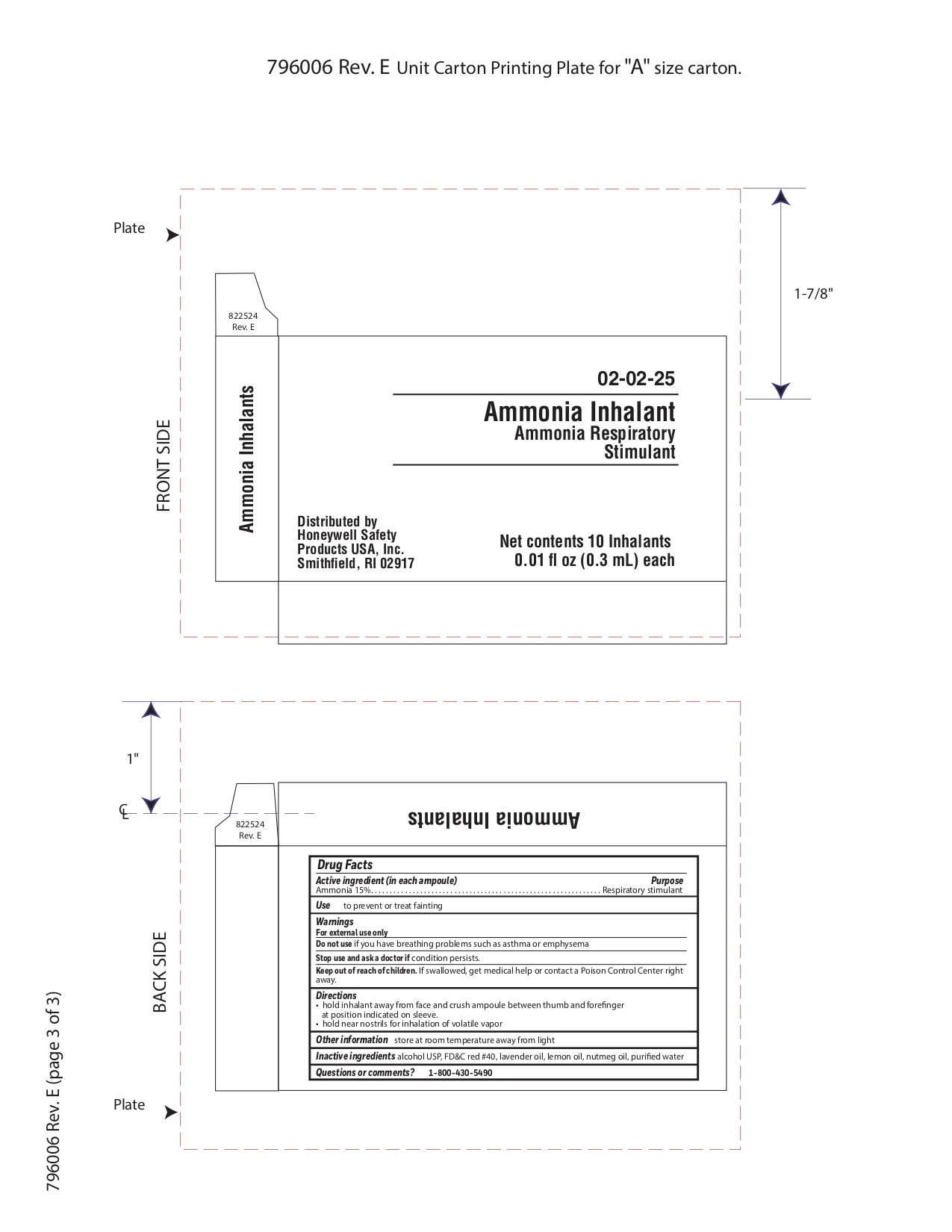
Ammonia Inhalent
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia Inhalent
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Burn Jel
Warnings
For external use only
Burn Jel
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water ...
Povidone Iodine Swab
Active ingredient
Povidone-iodine solution USP, 10% (equivalent to 1% titratable iodine)
Povidone Iodine Swab
Uses
- first aid to help prevent the risk of infection in minor cuts, scrapes, and burns
Povidone Iodine Swab
Directions
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
Povidone Iodine Swab
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
Povidone Iodine Swab
Inactive ingredients
citric acid, disodium phosphate,nonoxynol-9, sodium hydroxide, water
PAWS
USes
- for handwashing to decrease bacteria on skin whenever soap and water is not readily available
PAWS
Directions
- wet hands and wrists thoroughly for 15 seconds and allow to air dry
- always reseal after use
- children under 6 years of age should be supervised when using this product
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK
Other infprmation
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
4359
68WP3-01 Kit Contents
1 XTRA LRG 2" X 4" AWC
1 AMMONIA INHALANTS 10 PER
2 INSTANT COLD PACK 4" X 6"
1 1 OZ EYE WASH W/PADS & STRIPS
2 PVP IODINE WIPES 10 PER
2 ADHESIVE TAPE W/P 1/2"X 5 YD
2 ADH BDG, CLOTH, 1"X3", 16 PER
1 BK GZ 4.5"X4.1YD6PLY RL ST MSO
1 FIRST AID GUIDE ASHI
2 GAUZE CLEAN-WRAP BDGE N/S 2"
2 GAUZE CLEAN-WRAP BDGE N/S 3"
2 GAUZE CLEAN-WRAP BDGE N/S 4"
2 ABD COMBINE PAD 5" X 9"
2 ABD PADS 8"X10" STERILE
1 CO-FLEX BANDAGE 2"X 5YDS TAN
2 ELASTIC BANDAGE 3" X 4.5YD
1 ANTISEPTIC WIPES BZK CHL 20'S
1 BURN GEL 1/8 OZ 12/BAG
1 THERMOMETER DIGITAL FEVER
1 PENLIGHT DISPOSABLE EACH
1 SPLINTER FORCEP 4 1/2"
1 SCISSOR LISTER BDG S/S 7 1/4"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 LABEL, WATERPROOF KIT, LG
1 WATERPROOF CASE, LG, YEL
8 PR LRG NITRILE GLVES
3 GAUZE PADS 2"X2" 12PLY
6 GAUZE PADS 3"X3" 12PLY
3 GAUZE PADS 4"X4" 12PLY
1 ZIP-LOCK BAG 5" X 5" .002
2 TRIANG 37X37X52 UNIT
1 RESCUE BLANKET 1EA
1 CPR MSK,WPS,GLVS 1
| 4359 FIRST AID KIT
4359 first aid kit kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, INC (118768815) |