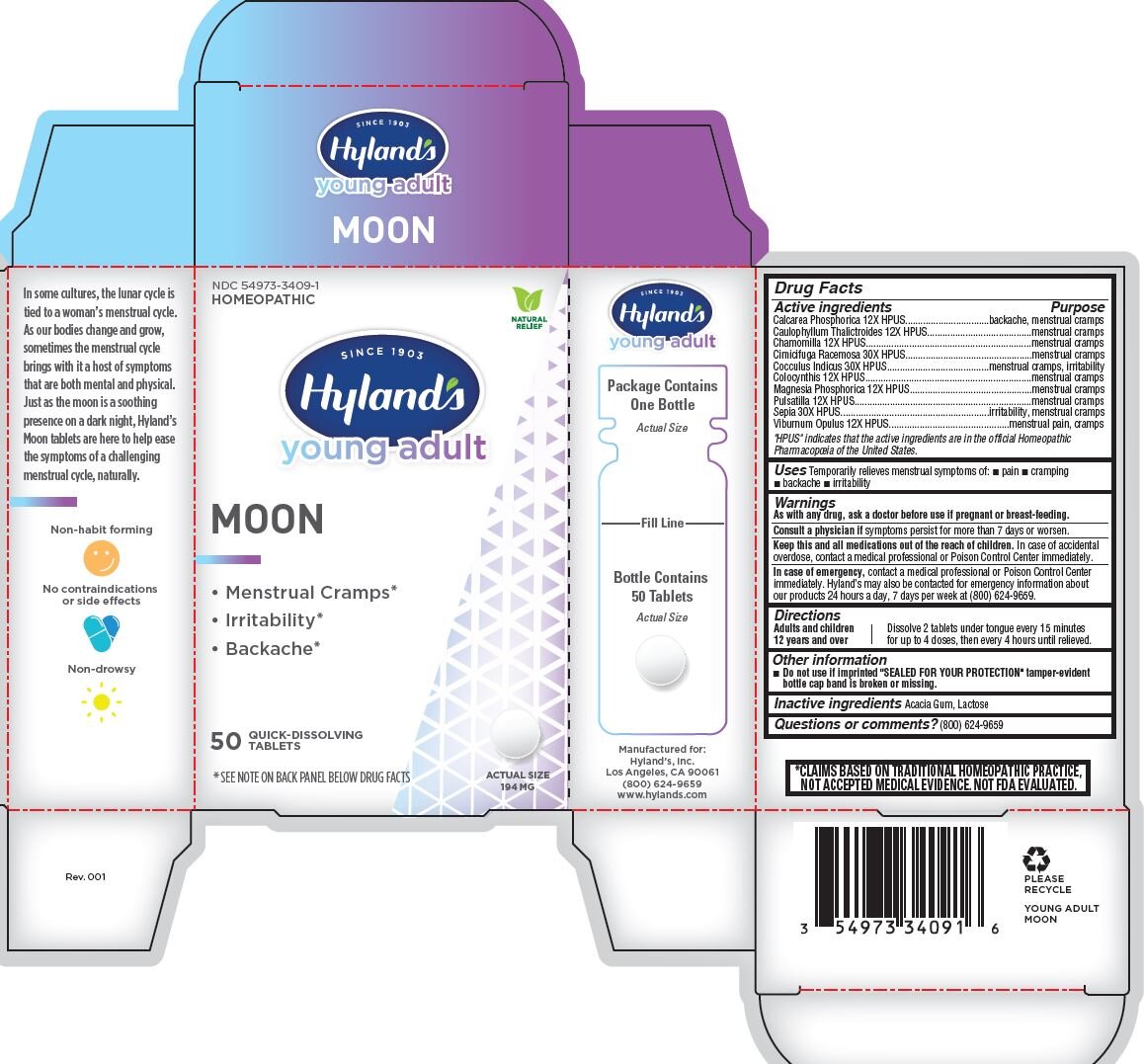
YOUNG ADULT MOON- magnesium phosphate, dibasic trihydrate, caulophyllum thalictroides root, matricaria chamomilla, viburnum opulus bark, anamirta cocculus seed, anemone pulsatilla, black cohosh, citrullus colocynthis fruit pulp, sepia officinalis juice and tribasic calcium phosphate tablet
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Hyland's Young Adult Moon
Drug Facts
Active ingredients
Calcarea Phosphorica 12X HPUS
Caulophyllum Thalictroides 12X HPUS
Chamomilla 12X HPUS
Cimicifuga Racemosa 30X HPUS
Cocculus Indicus 30X HPUS
Colocynthis 12X HPUS
Magnesia Phosphorica 12X HPUS
Pulsatilla 12X HPUS
Sepia 30X HPUS
Viburnum Opulus 12X HPUS
"HPUS" indicates that the active ingredients are in the official Homeopathic
Pharmacopoeia of the United States.
Warnings
As with any drug, ask a doctor before use if pregnant or breast-feeding.
Directions
|
Adults and children 12 years and over |
Dissolve 2 tablets under tongue every 15 minutes for up to 4 doses, then every 4 hours until relieved. |
Other information
Do not use if imprinted "SEALED FOR YOUR PROTECTION" tamper-evident bottle cap band is broken or missing.
| YOUNG ADULT MOON
magnesium phosphate, dibasic trihydrate, caulophyllum thalictroides root, matricaria chamomilla, viburnum opulus bark, anamirta cocculus seed, anemone pulsatilla, black cohosh, citrullus colocynthis fruit pulp, sepia officinalis juice and tribasic calcium phosphate tablet |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Hyland's (028570695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Homeopathic Company | 008316655 | manufacture(54973-3409) , pack(54973-3409) , label(54973-3409) | |