YOUNG ADULT CLEARAC- silicon dioxide, sulfur, potassium dichromate, activated charcoal, calcium sulfide, potassium bromide, arsenic trioxide and potassium chloride tablet
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
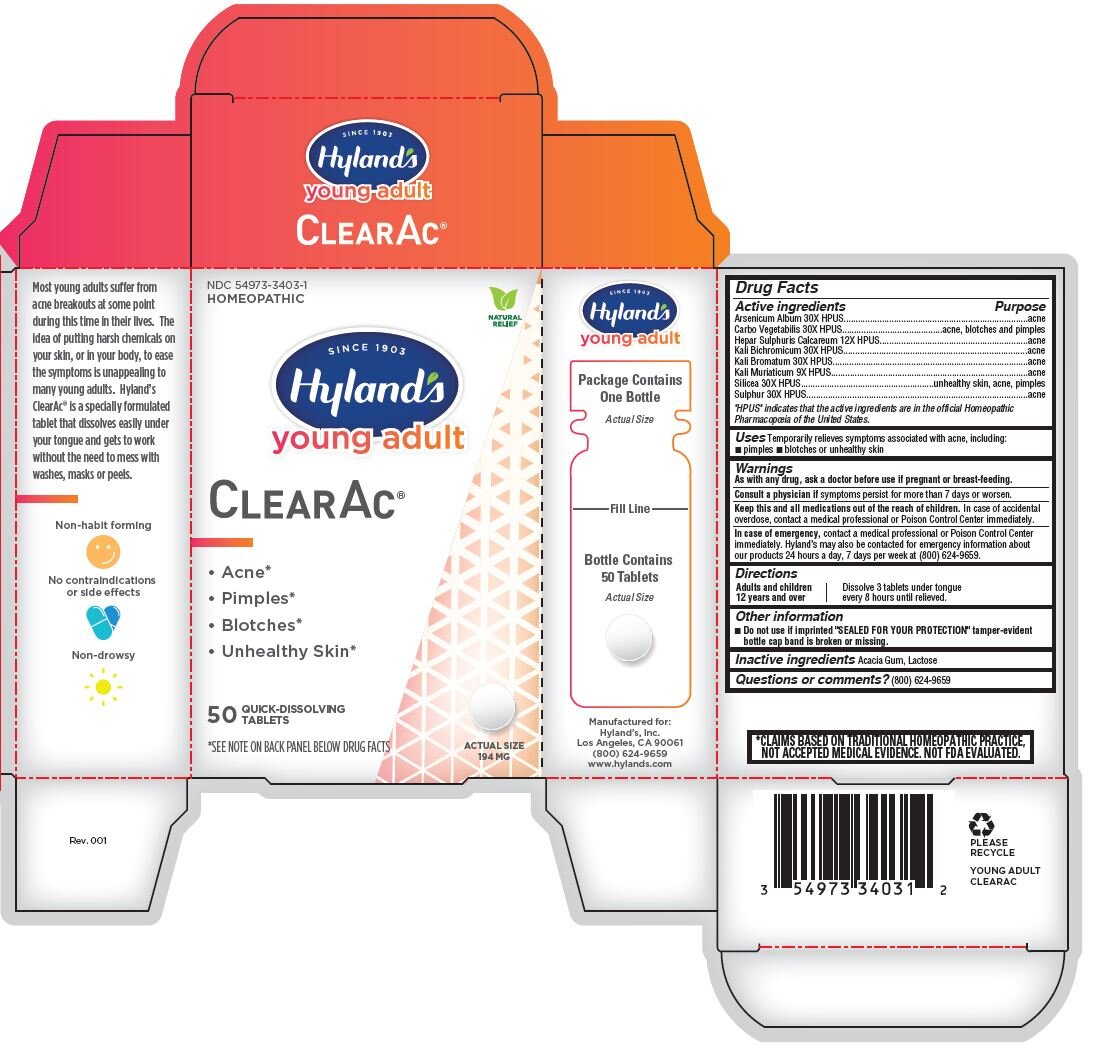
Hyland's Young Adult ClearAc
Uses
Temporarily relieves symptoms associated with acne, including: pimples, blotches or unhealthy skin
Warnings
As with any drug, ask a doctor before use if pregnant or breast-feeding.
Directions
|
Adults and children 12 years and over |
Dissolve 3 tablets under tongue every 8 hours until relieved. |
Other information
- Do not use if imprinted "SEALED FOR YOUR PROTECTION" tamper-evident bottle cap band is broken or missing.
| YOUNG ADULT CLEARAC
silicon dioxide, sulfur, potassium dichromate, activated charcoal, calcium sulfide, potassium bromide, arsenic trioxide and potassium chloride tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Hyland's (028570695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Homeopathic Company | 008316655 | manufacture(54973-3403) , pack(54973-3403) , label(54973-3403) | |
Revised: 12/2021
Document Id: d451be0a-b6ed-aee8-e053-2a95a90abe78
Set id: 8a2242a5-d1e3-2162-e053-2a95a90a9827
Version: 2
Effective Time: 20211229
Hyland's