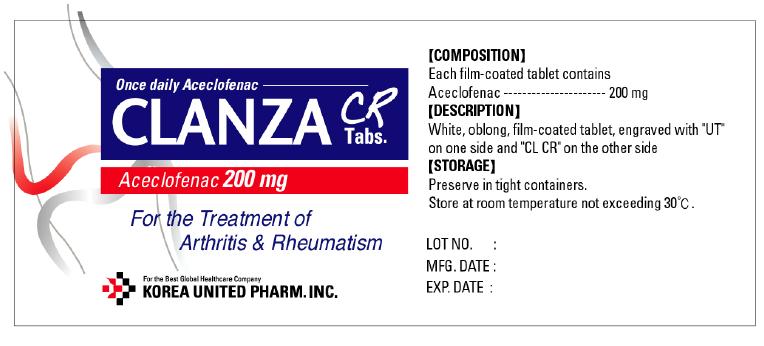
Label: CLANZA CR- aceclofenac tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 65697-450-20, 65697-450-21, 65697-450-22 - Packager: United Douglas Pharm., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated November 2, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- CONTRAINDICATIONS
- PRECAUTIONS
-
ADVERSE REACTIONS
ADVERSE REACTIONS
The majority of side effects observed have been reversible and of a minor nature and include gastro-intestinal disorders (dyspepsia, abdominal pain, nausea), rash, ruber, urticaria, symptoms of enuresis, headache, dizziness, and drowsiness. To report suspected adverse reactions, call 1-800-FDA-1088.
- GENERAL PRECAUTIONS
- DRUG INTERACTIONS
- PREGNANCY
- PEDIATRIC USE
-
OVERDOSAGE
OVERDOSAGE
There are no human data available on the consequences of CLANZA CR overdosage. If overdosage is observed, therapeutic measures should be taken according to symptoms; supportive and symptomatic treatment should be given for complications such as hypotension, gastro-intestinal irritation, respiratory depression, and convulsions.
- STORAGE AND HANDLING
- HOW SUPPLIED
- PATIENT PACKAGE INSERT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLANZA CR
aceclofenac tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65697-450 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Aceclofenac (UNII: RPK779R03H) (Aceclofenac - UNII:RPK779R03H) Aceclofenac 200 mg Inactive Ingredients Ingredient Name Strength Lactose Monohydrate (UNII: EWQ57Q8I5X) Cellulose, Microcrystalline (UNII: OP1R32D61U) Sodium Carbonate (UNII: 45P3261C7T) Colloidal Silicon Dioxide (UNII: ETJ7Z6XBU4) Crospovidone (UNII: 68401960MK) Poloxamer 407 (UNII: TUF2IVW3M2) Magnesium Stearate (UNII: 70097M6I30) Alcohol (UNII: 3K9958V90M) Hypromellose 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) Carbomer 941 (UNII: F68VH75CJC) Hypromellose 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) Alcohol (UNII: 3K9958V90M) Methylene Chloride (UNII: 588X2YUY0A) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Titanium Dioxide (UNII: 15FIX9V2JP) Ethylcelluloses (UNII: 7Z8S9VYZ4B) Diethyl Phthalate (UNII: UF064M00AF) Product Characteristics Color white Score no score Shape OVAL (Film-coated white oblong tablet) Size 15mm Flavor Imprint Code UT;CR;CT Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65697-450-20 1 in 1 PACKET 2 NDC:65697-450-22 10 in 1 CARTON 2 NDC:65697-450-21 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/12/2011 Labeler - United Douglas Pharm., Inc. (001444350) Registrant - United Douglas Pharm., Inc. (001444350) Establishment Name Address ID/FEI Business Operations United Douglas Pharm., Inc. 001444350 pack, label Establishment Name Address ID/FEI Business Operations Korea United Pharm Inc. 688016534 manufacture