THYROID RHYTHM- adrenalinum, calarea carbonica, calcarea fluorica, calcarea iodata, ferrum iodatum, fucus vesiculosus, iodium, kali iodatum, naturm muriaticum liquid
Alternative Pharmacy
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
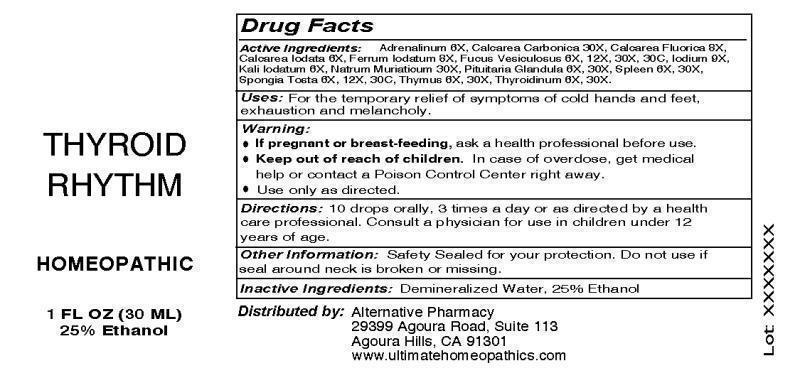
DRUG FACTS:
ACTIVE INGREDIENTS:
Adrenalinum 6X, Calcarea Carbonica 30X, Calcarea Fluorica 8X, Calcarea Iodata 6X, Ferrum Iodatum 8X, Fucus Vesiculosus 6X, 12X, 30X, 30C, Iodium 9X, Kali Iodatum 6X, Natrum Muriaticum 30X, Pituitaria Glandula (Bovine) 6X, 30X, Spleen (Bovine) 6X, 30X, Spongia Tosta 6X, 12X, 30C, Thymus (Bovine) 6X, 30X, Thyroidinum (Suis) 6X, 30X.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Use only as directed.
OTHER INFORMATION:
Safety Sealed for your protection. Do not use if seal around neck is broken or missing.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
10 drops orally, 3 times a day or as directed by a health care professional. Consult a physician for use in children under 12 years of age.
INDICATIONS:
For the temporary relief of symptoms of cold hands and feet, exhaustion and melancholy.
| THYROID RHYTHM
adrenalinum, calarea carbonica, calcarea fluorica, calcarea iodata, ferrum iodatum, fucus vesiculosus, iodium, kali iodatum, naturm muriaticum liquid |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Alternative Pharmacy (139900554) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(49304-0012) , api manufacture(49304-0012) , label(49304-0012) , pack(49304-0012) | |