Eyewash
Active ingredient
Sterile Water 99%
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Stop use and ask a doctor if
- you experience eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyewash
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
Eyewash
Questions
1-800-430-5490
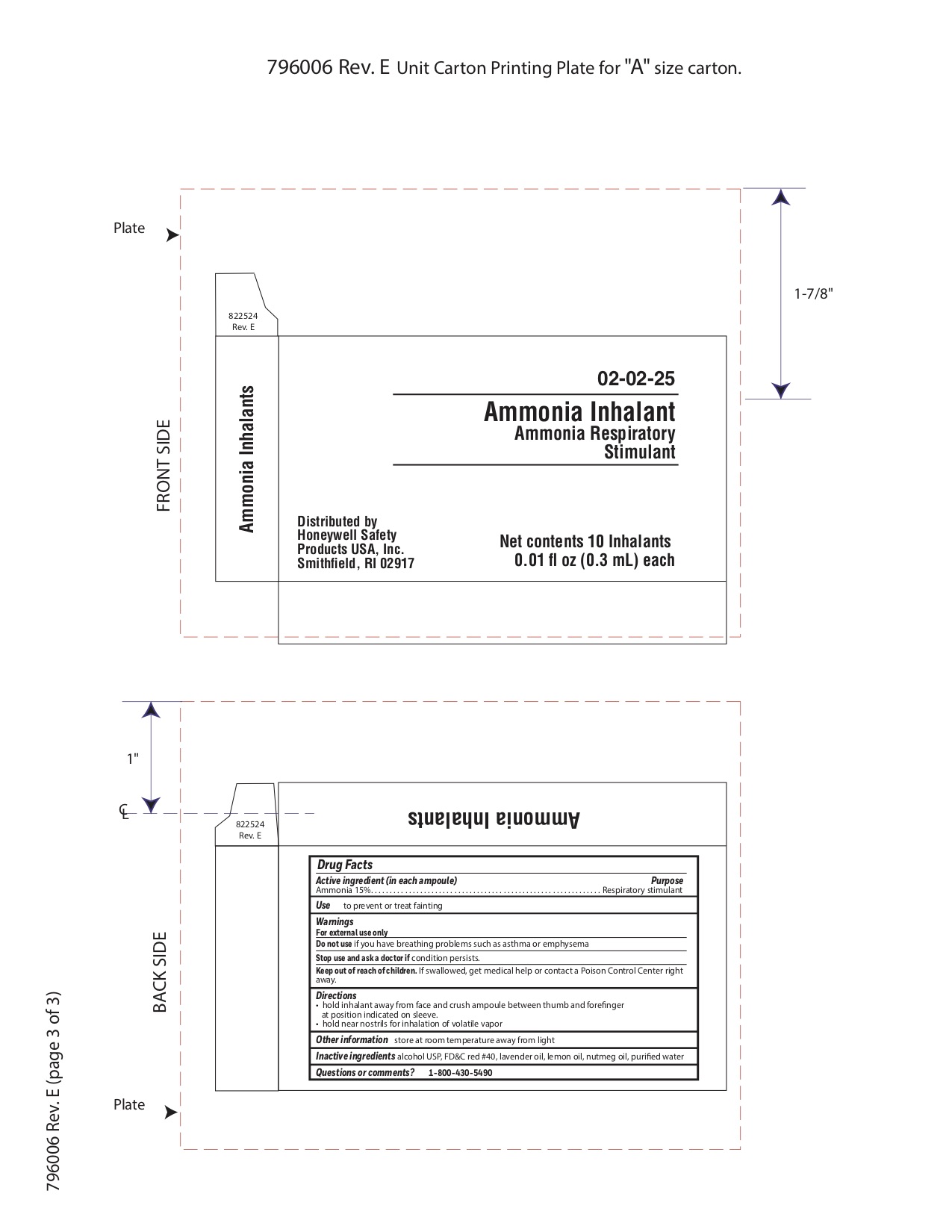
Ammonia
Active ingredient
Ammonia 15%
Ammonia
Purpose
Respiratory stimulant
Ammonia
Uses
- to prevent or treat fainting
Ammonia
Warnings
For external use only
Do not use
- if you have breathing problems such as asthma or emphysema
Stop use and ask a doctor if
Keep out of reach of children
- If swallowed get medical help or contact a Poison Control Center right away.
Ammonia
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia
Other information
- store at room temperature away from light
Ammonia
Inactive ingredient
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Ammonia
Questions or Comments?
1-800-430-5490
Pyrocaine
Active ingredient
Benzocaine 20%
Benzethonium chloride 0.2%
Pyrocaine
Purpose
Topical anesthetic
Topical antiseptic
Pyrocaine
Uses
For the temporary relief of pain and itching, and to help protect against skin infection in:
- minor burns
- minor skin irritations
- minor cuts and scrapes
- insect bites
- sunburns
Do not use
- in or near the eyes or over large portions of the body
- in case of deep or puncture wounds or on:
- raw surfaces
- blistered areas
- animal bites
- serious burns
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away
Pyrocaine
Warnings
For external use only
Flammable
- keep away from fire or flame
- contents under pressure
- do not puncture, incinerate or expose container to temperatures above 120 o F
Stop use and ask a doctor if
- If condition persists or worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days
Pyrocaine
Directions
- clean the affected area
- shake can well before using
- hold can 6 to 12 inches away from the affected area and spray liberally
- apply to affected area not more than 3 times daily
- for adult institutional use only
- not intended for use on children
Pyrocaine
Other information
- avoid inhaling
- use only as directed
- intentional misuse by deliberately concentrating and inhaling the contents may be harmful or fatal
Pyrocaine
Inactive ingredients
butane,dipropylene glycol, isobutane, propane
Burn Jel
Active ingredient
Lidocaine HCL 2.0%
Burn Jel
Purpose
External analgesic
Burn Jel
USes
- temporarily relieves pain due to minor burns
Do not use
- on large areas of the body, particularly over raw surfaces or blistered areas
Burn Jel
Warnings
For external use only
Stop use and ask a doctor if
- the condition gets worse
- symptoms persist for more than 7 days
- condition clears up and recurs within a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Burn Jel
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Other information
- store at room temperature
- do not use if opened or torn
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
Burn Jel
Questions
1-800-430-5490
PVP
Active ingredient
Povidone-iodine 10%
(equivalent to 1% titratable iodine)
PVP
Purpose
First aid antiseptic
PVP
Uses
- first aid antiseptic to help prevent infection in minor cuts, scrapes and burns
PVP
Warnings
For external use only.
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens or persists for more than 72 hours
- irritation and redness develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
PVP
Directions
- clean the affected area
- apply1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
- discard wipe after single use
PVP
Other information
- do not use on individuals who are allergic or sensitive to iodine
- store at controlled temperature 59-86ºF (15-30ºC)
- do not use if pouch is open or torn
PVP
Inactive ingredients
nonoxynol 9, water
PVP
Questions
1-800-430-5490
BZK
Active ingredient
Benzalkonium chloride 0.13% w/v
BZK
Purpose
First aid antiseptic
BZK
Uses
Antiseptic cleansing of face, hands, and body without soap and water
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- if irritation, redness or other symptoms develop
- the condition persists or gets worse
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
BZK
Directions
- tear open packet and use as a washcloth
BZK
Other information
store at room temperature 15
0 to 30
0 C (59
0 - 86
0 F)
do not reuse towelette
BZK
Inactive ingredient
water
BZK
Questions
1-800-430-5490
4345
Z019852 Kit Contents
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 TRIANGULAR BDG, NON-STERILE
2 INSTANT COLD PACK 4" X 6"
1 BUFFERED EYE WASH 1 OZ BTL
1 BANDAGE COMP, 3" OFFSET, 2 PER
1 BANDAGE COMP, 4" OFFSET, 1 PER
2 BURN JEL 1/8 OZ, 6 PER
1 WATER JEL DRESSING 4" X 4"
1 PVP IODINE WIPES 10 PER
1 ANTIMCRBL ANTSPTC TWLETTS
1 FIRST AID GUIDE ASHI
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 LBL CONTS 8"X8",CUSTOM ID B
1 KIT PP 24 UNIT FA
8 BLUE DETECTABLE KNUCKLE
1 ADHS TAPE .5"X2.5YD 2
1 PYRO-CAINE AERO 2/BX
1 SCISSOR & FORCEP 1 EA
2 BLUE 1"X3" BANDS 16'S
1 BLUE FINGERTIP "8" 8'S
Eyewash
Principal Display Panel

Ammonia
Principal Display Panel
Pyrocaine
Principal Display Panel

Burn Jel
Principal Display Panel

PVP
Principal Display Panel

BZK
Principal Display Panel

4345 Kit Label
Z019852







