3M NEXCARE COLD SORE TREATMENT- 3m nexcare cold sore treatment ointment
3M Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Nexcare™ Cold Sore Treatment
- Unique combination of ingredients
- Helps relieve cold sore symptoms
- Enriched with tea tree oil and Vitamin E
- Helps get you back to living comfortably
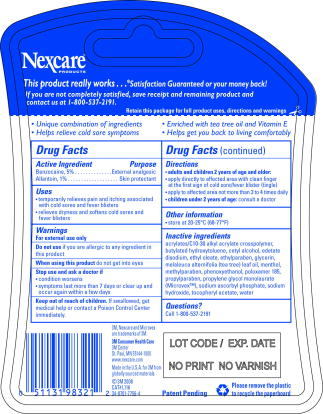
Drug Facts
Uses
- temporarily relieves pain and itching associated with cold sores and fever blisters
- relieves dryness and softens cold sores and fever blisters
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- adults and children 2 years of age and older:
- apply directly to affected area with clean finger at the first sign of cold sore/fever blister (tingle)
- apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: consult a doctor
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, butylated hydroxytoluene, cetyl alcohol, edetate disodium, ethyl oleate, ethylparaben, glycerin, melaleuca alternifolia (tea tree) leaf oil, menthol, methylparaben, phenoxyethanoi, poloxamer 185, propylparaben, propylene glycol monolaurate (Microvex™), sodium ascorbyl phosphate, sodium hydroxide, tocopheryl acetate, water
| 3M NEXCARE COLD SORE TREATMENT
3m nexcare cold sore treatment ointment |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - 3M Company (006173082) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Celeste Contract Packaging | 829739833 | MANUFACTURE(17518-054) | |