FLUROX FLU- anas barbariae, astragalus, echinacea ang, hydrastis, tauroxicum, zinc phos liquid
FindCure.org
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
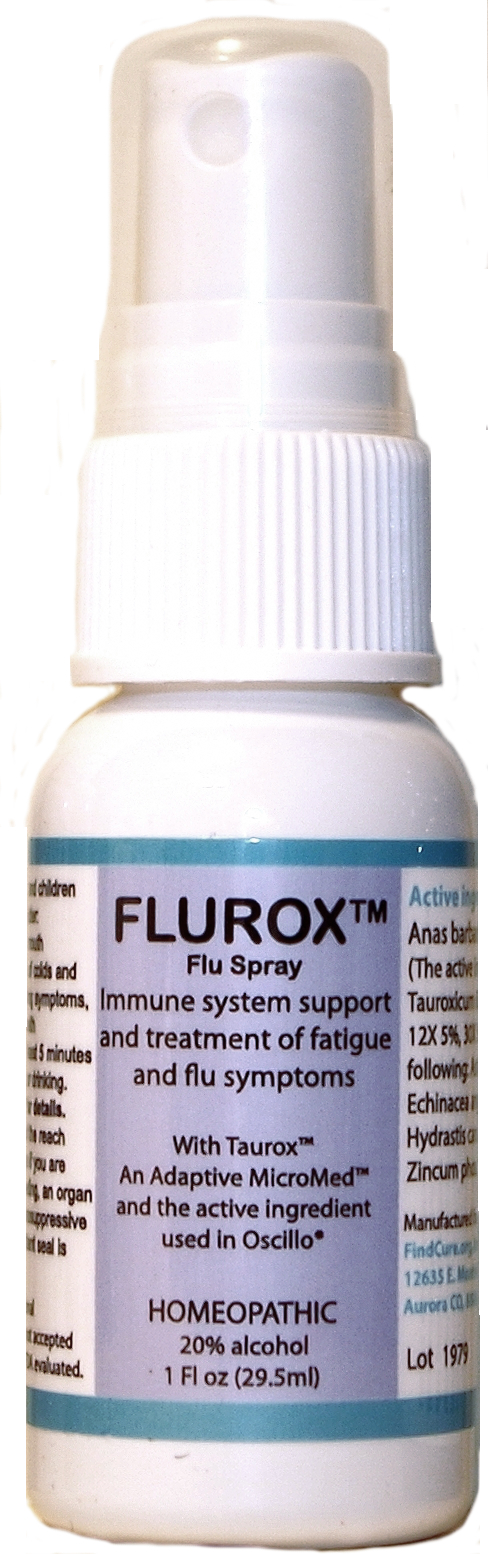
Flurox Flu Spray
Active Ingredients
Anas Barbariae 200CK 61.7%;
Tauroxicum 6X 5.8%
2.5% for each of the following:
Astragalus 6X, 12X, 30X
Echinacea ang 6X, 12X, 30X
Hydrastis 6X, 12X, 30X
Tauroxicum 12X, 30X
Zinc phos, 12X, 30X
Directions
Directions for adults and children 15 years of age and older: Take 2 to 4 sprays in mouth weekly for prevention of colds and flu, and take daily during symptoms, or as directed by a health professional. Take at least 5 minutes before or after eating or drinking. See package insert for details.
Warnings
Warnings
Keep out of reach of children. Do not use if you are pregnant or breast feeding, an organ recipient, using immunosuppressive drugs, or if the tamper-evident seal is broken or missing. See package insert for details.
| FLUROX FLU
anas barbariae, astragalus, echinacea ang, hydrastis, tauroxicum, zinc phos liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - FindCure.org (026023141) |