UNDECYLENIC ACID- antifungal solution liquid
Rite Aid Corporation
----------
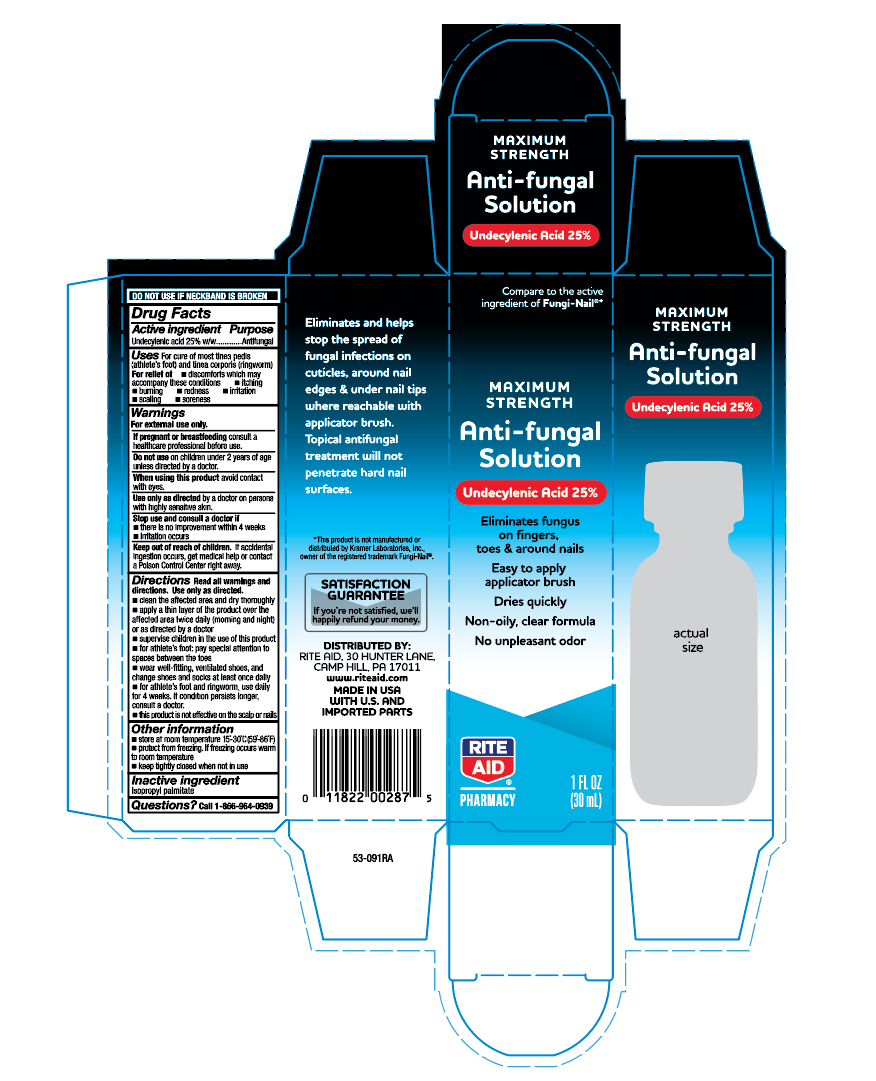
Rite Aid Antifungal Nail Solution
Uses
For cure of most tinea pedis (athete's foot) and tinea corporis (ringworm)
For relief of:
- discomforts which may accompany these conditons
- itching
- burning
- redness
- irritation
- scaling
- soreness
Directions
Read all warnings and directions. Use only as directed.
- clean the affected area and dry thoroughly
- apply a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between the toes
- wear well-fitting, ventilated shoes, and changes shoes and socks atleast once daily
- for athlete's foot and ringworm, use daily for 4 weeks. If condition persists longer, consult a doctor.
- this product is not effective on the scalp or nails
| UNDECYLENIC ACID
antifungal solution liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Rite Aid Corporation (014578892) |
Revised: 2/2024
Document Id: 115f0ebd-2970-45f2-e063-6394a90ae7e2
Set id: 8834939f-3f26-4ce0-9269-fd3e781acc67
Version: 11
Effective Time: 20240214
Rite Aid Corporation