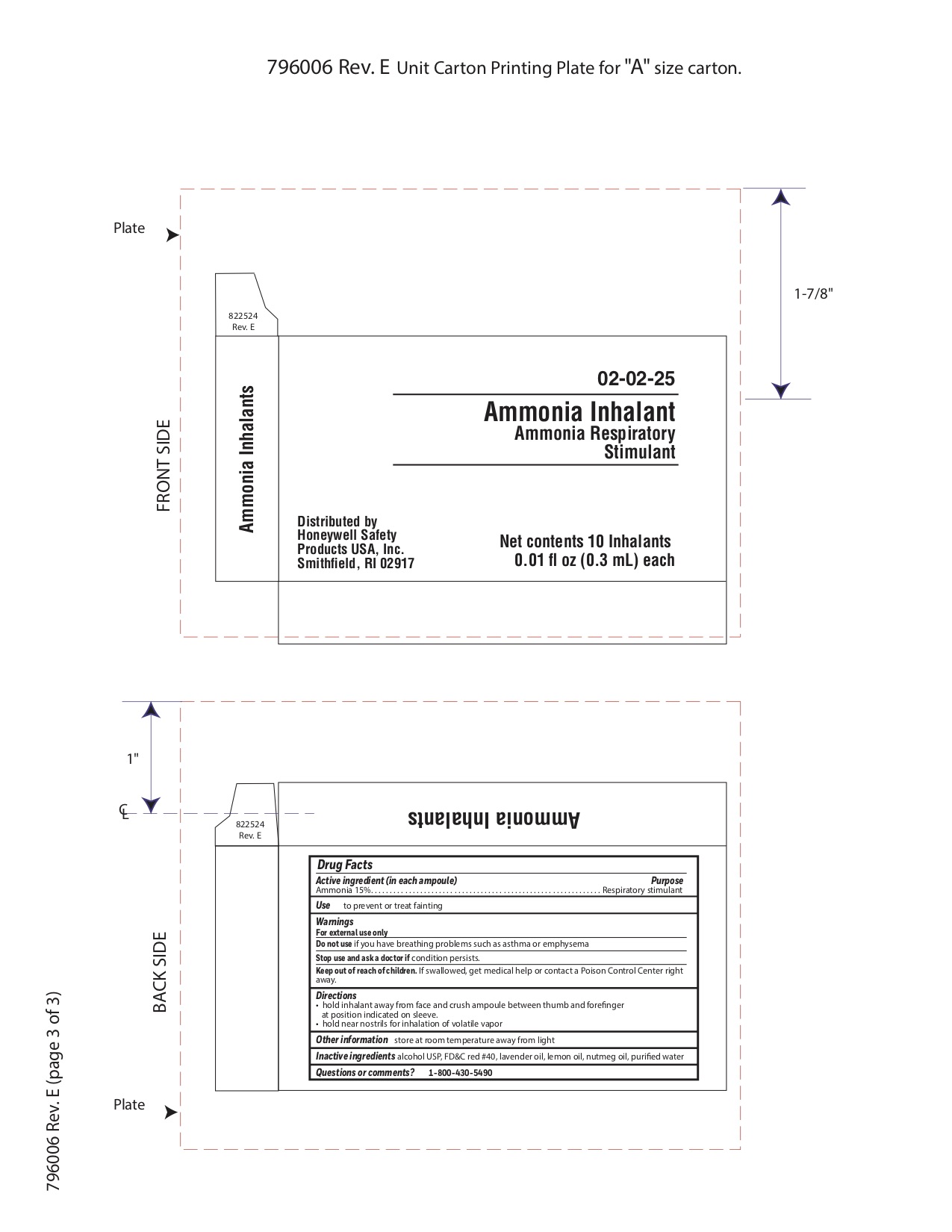
Active ingredient
Ammonia 15%
Purpose
Respiratory stimulant
Uses
to prevent or treat fainting
Warnings
For external use only
Do not use
- if you have asthma or emphysema
Stop use and ask a doctor if
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Other information
store at room temperature away from light
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Questions or Comments
1-800-430-5490
PVP
Active ingredient
Povidone-iodine solution USP, 10% (equivalent to 1% titratable iodine)
PVP
Purpose
First aid antisepti
PVP
Uses
- first aid to help prevent the risk of infection in minor cuts, scrapes, and burn
PVP
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body
- on individuals who are allergic or sensitive to iodine
Ask a doctor before use if you have
- deep or puncture wounds,
- animal bites
- serious burns
When using this product
- do not use longer than one wek unless directed by a doctor
Stop use and ask a doctor if
- conditions persists or gets worse
- irritation and redness develops
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away
PVP
Directioons
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
PVP
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
PVP
Inactive ingredients
citric acid, disodium phosphate,nonoxynol-9, sodium hydroxide, water
PVP
Questions and Comments?
1-800-430-5490
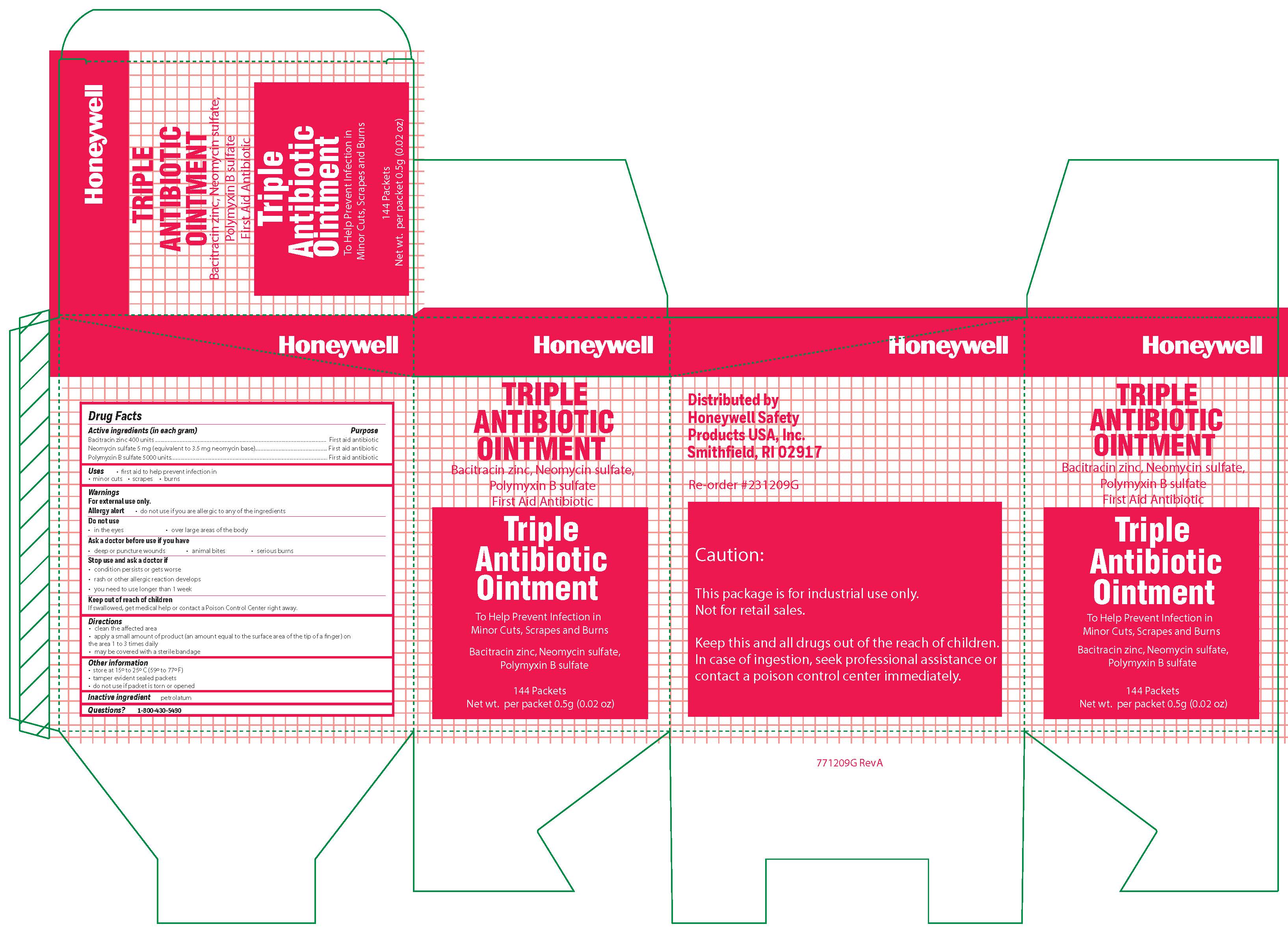
Triple
Active ingredient
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Purpose
First aid antibiotic
First aid antibiotic
First aid antibiotic
Triple
Uses
first aid to help prevent infection in:
Triple
Warnings
For external use only
Allergy alert:
do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- the condition persists or gets worse
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15
0 to 25
0 C (59
0 to 77
0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
Triple
Inactive ingredient
petrolatum
Triple
Questions?
1-800-430-5490
BZK Wipe
Active ingredient
Benzalkonium chloride 0.13% w/v
BZK Wipe
Purpose
First aid antiseptic
BzK Wipe
Uses
Antiseptic cleansing of face, hands, and body without soap and water
BZK Wipe
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- if irritation, redness or other symptoms develop
- the condition persists or gets worse
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
BZK Wipe
Directions
tear open packet and use as a washcloth
BZK Wipe
Other information
- store at room temperature 15
0 to 30
0 C (59
0 - 86
0 F)
- do not reuse towelette
BZK Wipe
Other information
water
BZK Wipe
Questions
1-800-430-5490
4135
SF00001642 Kit Contents
1 3/4 X 3 PLAS 100/BOX
1 TRIPLE ANTIBIOTIC 10 PER
2 INSTANT COLD PACK 4" X 6"
1 1 OZ EYE WASH W/PADS & STRIPS
4 PVP IODINE WIPES 10 PER
1 ANTIMCRBL ANTSPTC TWLETTS
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 FIRST AID GUIDE ASHI
6 GAUZE CLEAN-WRAP BDGE N/S 2"
2 ABD COMBINE PAD 5" X 9"
1 GZE PADS STERILE 2"X 2" 25'S
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 ZIP BAG FOR 50 ECONO
1 KIT STL BULK MEDIUM
1 TRI BNDG NON WOVEN 40"X40"X56"
Principal Display Panel
PVP
Principal Display Panel

Triple
Principal Display Panel
BZK Wipe
Principal Display Panel

4135 Kit Label
SF00001642

Honeywell Safety Products USA, Inc.




