WOMENS GENTLE LAXATIVE- bisacodyl tablet, coated
FRED'S, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Freds 44-326-Delisted
Uses
-
for gentle, overnight relief of occasional constipation (irregularity)
-
this product generally produces bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if you have
-
abdominal pain
-
nausea
-
vomiting
-
sudden change in bowel movements that persist over a period of 2 weeks
Directions
-
do not chew or crush tablets
-
do not take within 1 hour after taking an antacid or milk
| adults and children over 12 years of age | oral dosage is 1 to 3 tablets in a single or divided daily dose |
| children 6 to under 12 years of age | oral dosage is 1 tablet in a single daily dose |
| children under 6 years of age | ask a doctor |
Other information
-
store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
-
protect from moisture
-
see end flap for expiration date and lot number
Inactive ingredients
acacia, black iron oxide, calcium carbonate, carnauba wax, corn starch, D&C red #27 aluminum lake, FD&C blue #2 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, lactose, magnesium stearate, methylparahydroxy benzoate, polydextrose, polyethylene glycol, polyvinyl acetate phthalate, povidone, propylene glycol, propylparahydroxy benzoate, shellac glaze, silica, sodium alginate, sodium benzoate, sodium bicarbonate, stearic acid, sucrose, talc, titanium dioxide, triacetin, triethyl citrate
Principal Display Panel
FRED'S®
Stimulant Laxative
Women's
Gentle Laxative
Bisacodyl 5 mg
Gentle, Dependable Overnight Relief
30 Enteric Coated Tablets
Tested Against The Active Ingredient In: CORRECTOL®*
*This product is not manufactured or distributed Schering-Plough Healthcare Products, owner of the registered trademark Correctol®.
50844 REV0410E32601
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF A BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Fred's 44-326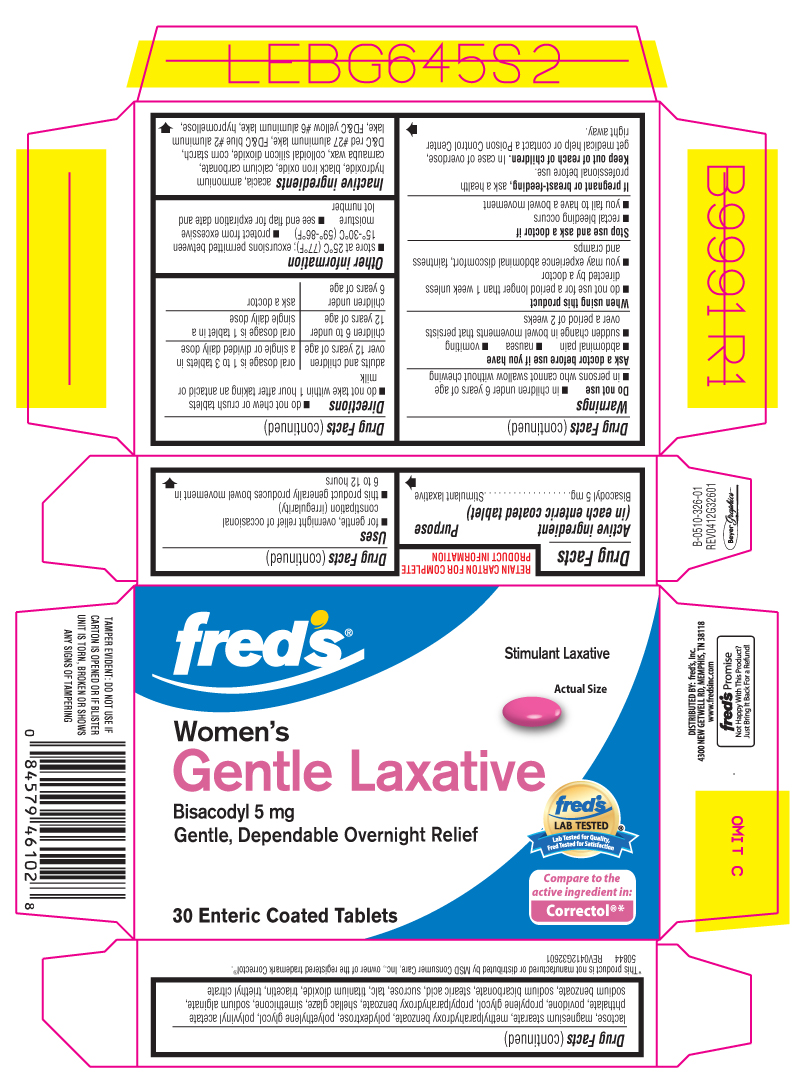
| WOMENS GENTLE LAXATIVE
bisacodyl tablet, coated |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - FRED'S, INC. (005866116) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(55315-326) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(55315-326) | |