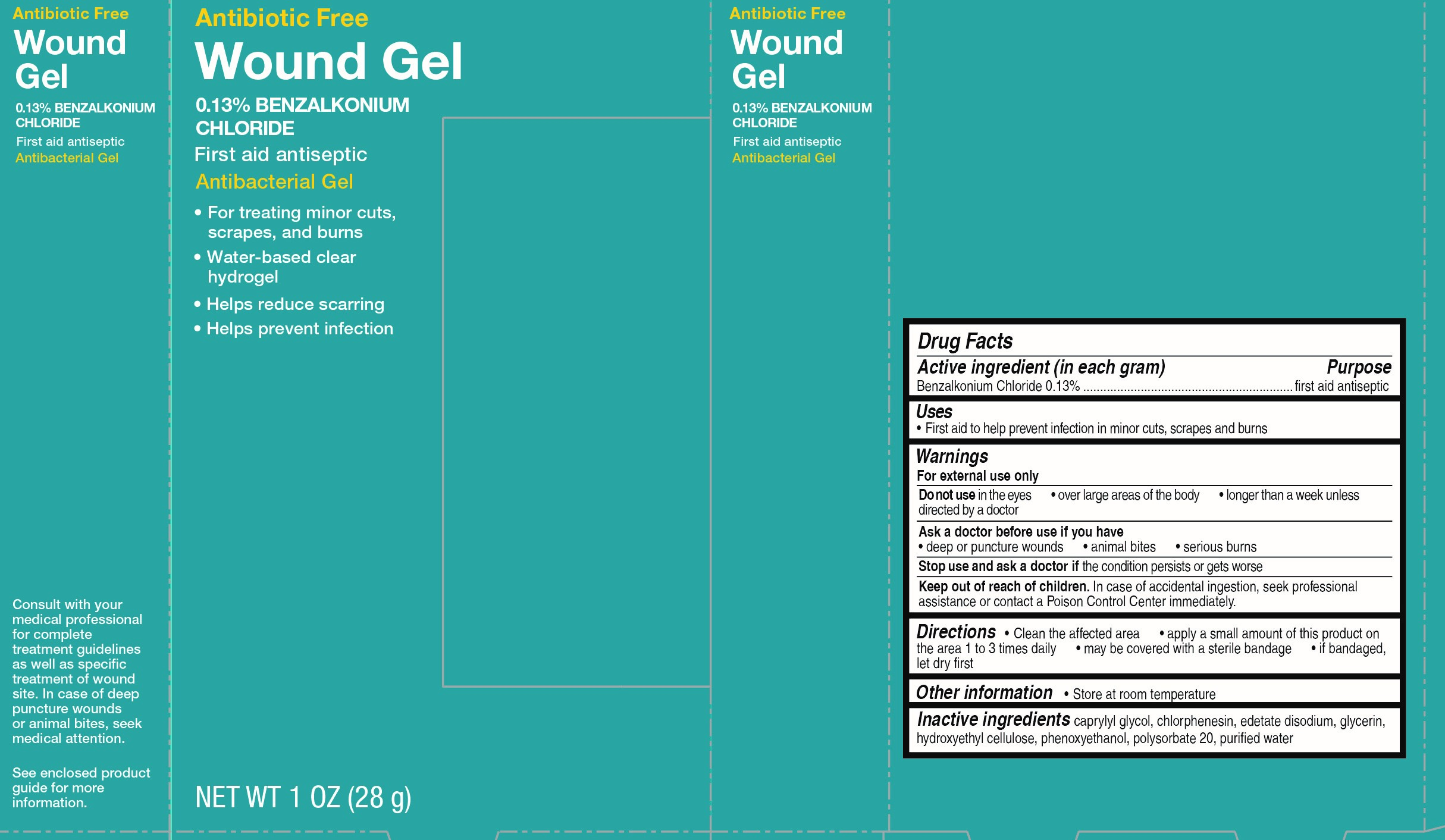
ANTIBIOTIC FREE WOUND GEL- benzalkonium chloride gel
ASO LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Antibiotic Free Wound Gel
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
- Clean the affected area
- apply a small amount of this product on the area 1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
| ANTIBIOTIC FREE WOUND GEL
benzalkonium chloride gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ASO LLC (152793493) |
Revised: 2/2021
Document Id: ba4c3694-1b15-0c13-e053-2995a90af6d5
Set id: 87212c08-c81c-86c1-e053-2a91aa0af238
Version: 3
Effective Time: 20210201
ASO LLC