Label: imodium- Loperamide Hydrochloride capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 50458-400-10 - Packager: McNeil Consumer Healthcare
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated February 26, 2008
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
-
DESCRIPTION
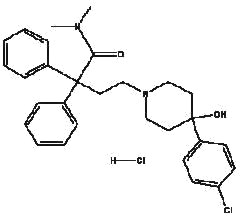
IMODIUM® (loperamide hydrochloride), 4-(p-chlorophenyl)-4-hydroxy-N,N-dimethyl-a,a-diphenyl-1-piperidinebutyramide monohydrochloride, is a synthetic antidiarrheal for oral use.
IMODIUM® is available in 2mg capsules.
The inactive ingredients are: Lactose, cornstarch, talc, and magnesium stearate. IMODIUM® capsules contain FD&C Yellow No. 6.
-
CLINICAL PHARMACOLOGY
In vitro and animal studies show that IMODIUM® (loperamide hydrochloride) acts by slowing intestinal motility and by affecting water and electrolyte movement through the bowel. Loperamide binds to the opiate receptor in the gut wall. Consequently, it inhibits the release of acetylcholine and prostaglandins, thereby reducing peristalsis, and increasing intestinal transit time. Loperamide increases the tone of the anal sphincter, thereby reducing incontinence and urgency.
In man, IMODIUM® prolongs the transit time of the intestinal contents. It reduces daily fecal volume, increases the viscosity and bulk density, and diminishes the loss of fluid and electrolytes. Tolerance to the antidiarrheal effect has not been observed. Clinical studies have indicated that the apparent elimination half-life of loperamide in man is 10.8 hours with a range of 9.1 - 14.4 hours. Plasma levels of unchanged drug remain below 2 nanograms per mL after the intake of a 2mg capsule of IMODIUM®. Plasma levels are highest approximately five hours after administration of the capsule and 2.5 hours after the liquid. The peak plasma levels of loperamide were similar for both formulations. Elimination of loperamide mainly occurs by oxidative N-demethylation. Cytochrome P450 (CYP450) isozymes, CYP2C8 and CYP3A4, are thought to play an important role in loperamide N-demethylation process since quercetin (CYP2C8 inhibitor) and ketoconazole (CYP3A4 inhibitor) significantly inhibited the N-demethylation process in vitro by 40% and 90%, respectively. In addition, CYP2B6 and CYP2D6 appear to play a minor role in loperamide N-demethylation. Excretion of the unchanged loperamide and its metabolites mainly occurs through the feces. In those patients in whom biochemical and hematological parameters were monitored during clinical trials, no trends toward abnormality during IMODIUM® therapy were noted. Similarly, urinalyses, EKG and clinical ophthalmological examinations did not show trends toward abnormality.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
IMODIUM is contraindicated in patients with a known hypersensitivity to loperamide hydrochloride or to any of the excipients.
IMODIUM is contraindicated in patients with abdominal pain in the absence of diarrhea.
IMODIUM is not recommended in infants below 24 months of age.
IMODIUM should not be used as the primary therapy:
- -
- in patients with acute dysentery, which is characterized by blood in stools and high fever,
- -
- in patients with acute ulcerative colitis,
- -
- in patients with bacterial enterocolitis caused by invasive organisms including Salmonella, Shigella, and Campylobacter,
- -
- in patients with pseudomembranous colitis associated with the use of broad-spectrum antibiotics.
-
WARNINGS
Fluid and electrolyte depletion often occur in patients who have diarrhea. In such cases, administration of appropriate fluid and electrolytes is very important. The use of IMODIUM® does not preclude the need for appropriate fluid and electrolyte therapy.
In general, IMODIUM should not be used when inhibition of peristalsis is to be avoided due to the possible risk of significant sequelae including ileus, megacolon and toxic megacolon. IMODIUM must be discontinued promptly when constipation, abdominal distention or ileus develop.
Treatment of diarrhea with IMODIUM is only symptomatic. Whenever an underlying etiology can be determined, specific treatment should be given when appropriate (or when indicated).
Patients with AIDS treated with IMODIUM for diarrhea should have therapy stopped at the earliest signs of abdominal distention. There have been isolated reports of toxic megacolon in AIDS patients with infectious colitis from both viral and bacterial pathogens treated with loperamide hydrochloride.
IMODIUM® should be used with special caution in young children because of the greater variability of response in this age group. Dehydration, particularly in younger children, may further influence the variability of response to IMODIUM®.
-
PRECAUTIONS
General
Extremely rare allergic reactions including anaphylaxis and anaphylactic shock have been reported. In acute diarrhea, if clinical improvement is not observed in 48 hours, the administration of IMODIUM® (loperamide hydrochloride) should be discontinued and patients should be advised to consult their physician. Although no pharmacokinetic data are available in patients with hepatic impairment, IMODIUM should be used with caution in such patients because of reduced first pass metabolism. Patients with hepatic dysfunction should be monitored closely for signs of CNS toxicity. No pharmacokinetic data are available in patients with renal impairment. Since it has been reported that the majority of the drug is metabolized and metabolites or the unchanged drug is excreted mainly in the feces, dosage adjustments in patients with renal impairment are not required. No formal studies have been conducted to evaluate the pharmacokinetics of loperamide in elderly subjects. However, in two studies that enrolled elderly patients, there were no major differences in the drug disposition in elderly patients with diarrhea relative to young patients.
Information for Patients
Patients should be advised to check with their physician if their diarrhea does not improve in 48 hours or if they note blood in their stools, develop a fever or develop abdominal distention. Tiredness, dizziness, or drowsiness may occur in the setting of diarrheal syndromes treated with IMODIUM. Therefore, it is advisable to use caution when driving a car or operating machinery. (see Adverse Reactions).
Drug Interactions
Nonclinical data have shown that loperamide is a P-glycoprotein substrate. Concomitant administration of loperamide (16 mg single dose) with a 600 mg single dose of either quinidine, or ritonavir, both of which are P-glycoprotein inhibitors, resulted in a 2- to 3- fold increase in loperamide plasma levels. Due to the potential for enhanced central effects when loperamide is coadministered with quinidine and with ritonavir, caution should be exercised when loperamide is administered at the recommended dosages (2 mg, up to 16 mg maximum daily dose) with P-glycoprotein inhibitors.
When a single 16-mg dose of loperamide is coadministered with a 600 mg single dose of saquinavir, loperamide decreased saquinavir exposure by 54%, which may be of clinical relevance due to reduction of therapeutic efficacy of saquinavir. The effect of saquinavir on loperamide is of less clinical significance. Therefore, when loperamide is given with saquinavir, the therapeutic efficacy of saquinavir should be closely monitored.
Carcinogenesis, mutagenesis, impairment of fertility
In an 18-month rat study with oral doses up to 40 mg/kg/day (21 times the maximum human dose of 16 mg/day, based on a body surface area comparison), there was no evidence of carcinogenesis.
Loperamide was not genotoxic in the Ames test, the SOS chromotest in E. coli, the dominant lethal test in female mice, or the mouse embryo cell transformation assay.
Fertility and reproductive performance was evaluated in rats using oral doses of 2.5, 10, and 40 mg/kg/day (females only) in a second study. Oral administration of 20 mg/kg/day (approximately 11 times the human dose based on a body surface area comparison) and higher produced strong impairment of female fertility. Treatment of female rats with up to 10 mg/kg/day by mouth (approximately 5 times the human dose based on a body surface area comparison) had no effect on fertility. Treatment of male rats with 40 mg/kg/day by mouth (approximately 21 times the human dose based on a body surface area comparison) produced impairment of male fertility, whereas administration of up to 10 mg/kg/day (approximately 5 times the human dose based on a body surface area comparison) had no effect.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Teratology studies have been performed in rats using oral doses of 2.5, 10, and 40 mg/kg/day, and in rabbits using oral doses of 5, 20, and 40 mg/kg/day. These studies have revealed no evidence of impaired fertility or harm to the fetus at doses up to 10 mg/kg/day in rats (5 times the human dose based on body surface area comparison) and 40 mg/kg/day in rabbits (43 times the human dose based on body surface area comparison). Treatment of rats with 40 mg/kg/day by mouth (21 times the human dose based on a body surface area comparison) produced marked impairment of fertility. The studies produced no evidence of teratogenic activity. There are no adequate and well-controlled studies in pregnant women. Loperamide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Small amounts of loperamide may appear in human breast milk. Therefore, IMODIUM is not recommended during breast-feeding.
Pediatric Use
See the "Warnings" Section for information on the greater variability of response in this age group. In case of accidental overdosage of IMODIUM® by children, see "Overdosage" Section for suggested treatment.
-
ADVERSE REACTIONS
Clinical Trial Data
The adverse effects reported during clinical investigations of IMODIUM® (loperamide hydrochloride) are difficult to distinguish from symptoms associated with the diarrheal syndrome. Adverse experiences recorded during clinical studies with IMODIUM® were generally of a minor and self-limiting nature. They were more commonly observed during the treatment of chronic diarrhea.
The adverse events reported are summarized irrespective of the causality assessment of the investigators.
1) Adverse events from 4 placebo-controlled studies in patients with acute diarrhea
The adverse events with an incidence of 1.0% or greater, which were reported at least as often in patients on loperamide hydrochloride as on placebo, are presented in the table below.
Acute Diarrhea Loperamide Hydrochloride Placebo No. of treated patients 231 236 Gastrointestinal AE% Constipation 2.6% 0.8% The adverse events with an incidence of 1.0% or greater, which were more frequently reported in patients on placebo than on loperamide hydrochloride, were: dry mouth, flatulence, abdominal cramp and colic.
2) Adverse events from 20 placebo-controlled studies in patients with chronic diarrhea
The adverse events with an incidence of 1.0% or greater, which were reported at least as often in patients on loperamide hydrochloride as on placebo, are presented below in the table below.
Chronic Diarrhea Loperamide Hydrochloride Placebo No. of treated patients 285 277 Gastrointestinal AE% Constipation 5.3% 0.0% Central and peripheral nervous system AE% Dizziness 1.4% 0.7% The adverse events with an incidence of 1.0% or greater, which were more frequently reported in patients on placebo than on loperamide hydrochloride were: nausea, vomiting, headache, meteorism, abdominal pain, abdominal cramp and colic.
3) Adverse events from seventy-six controlled and uncontrolled studies in patients with acute or chronic diarrhea
The adverse events with an incidence of 1.0% or greater in patients from all studies are given in the table below.
Acute Diarrhea Chronic Diarrhea All Studies * - *
- All patients in all studies, including those in which it was not specified if the adverse events occurred in patients with acute or chronic diarrhea.
No. of treated patients 1913 1371 3740 Gastrointestinal AE% Nausea 0.7% 3.2% 1.8% Constipation 1.6% 1.9% 1.7% Abdominal cramps 0.5% 3.0% 1.4% Post –marketing experience
The following adverse events have been reported:
Skin and subcutaneous tissue disorders
Rash, pruritus, urticaria, angioedema, and extremely rare cases of bullous eruption including erythema multiforme, Stevens-Johnson syndrome and Toxic Epidermal Necrolysis have been reported with use of IMODIUM
Immune system disorders
Isolated occurrences of allergic reactions and in some cases severe hypersensitivity reactions including anaphylactic shock and anaphylactoid reactions have been reported with the use of IMODIUM.Gastrointestinal disorders
Dry mouth, abdominal pain, distention or discomfort, nausea, vomiting, flatulence, dyspepsia, constipation, paralytic ileus, megacolon, including toxic megacolon (see Contraindications and Warnings).Renal and urinary disorders
Urinary retentionNervous system disorders
Drowsiness, dizzinessGeneral disorders and administrative site conditions
TirednessA number of the adverse events reported during the clinical investigations and post-marketing experience with loperamide are frequent symptoms of the underlying diarrheal syndrome (abdominal pain/discomfort, nausea, vomiting, dry mouth, tiredness, drowsiness, dizziness, constipation, and flatulence). These symptoms are often difficult to distinguish from undesirable drug effects.
-
DRUG ABUSE AND DEPENDENCE
Abuse
A specific clinical study designed to assess the abuse potential of loperamide at high doses resulted in a finding of extremely low abuse potential.
Dependence
Studies in morphine-dependent monkeys demonstrated that loperamide hydrochloride at doses above those recommended for humans prevented signs of morphine withdrawal. However, in humans, the naloxone challenge pupil test, which when positive indicates opiate-like effects, performed after a single high dose, or after more than two years of therapeutic use of IMODIUM® (loperamide hydrochloride), was negative. Orally administered IMODIUM® (loperamide formulated with magnesium stearate) is both highly insoluble and penetrates the CNS poorly.
-
OVERDOSAGE
In cases of overdosage, (including relative overdose due to hepatic dysfunction), urinary retention, paralytic ileus and CNS depression may occur. Children may be more sensitive to CNS effects than adults. Clinical trials have demonstrated that a slurry of activated charcoal administered promptly after ingestion of loperamide hydrochloride can reduce the amount of drug which is absorbed into the systemic circulation by as much as ninefold. If vomiting occurs spontaneously upon ingestion, a slurry of 100 gms of activated charcoal should be administered orally as soon as fluids can be retained.
If vomiting has not occurred, gastric lavage should be performed followed by administration of 100 gms of the activated charcoal slurry through the gastric tube. In the event of overdosage, patients should be monitored for signs of CNS depression for at least 24 hours.
If symptoms of overdose occur, naloxone can be given as an antidote. If responsive to naloxone, vital signs must be monitored carefully for recurrence of symptoms of drug overdose for at least 24 hours after the last dose of naloxone.
In view of the prolonged action of loperamide and the short duration (one to three hours) of naloxone, the patient must be monitored closely and treated repeatedly with naloxone as indicated. Since relatively little drug is excreted in the urine, forced diuresis is not expected to be effective for IMODIUM® (loperamide hydrochloride) overdosage.
In clinical trials an adult who took three 20mg doses within a 24 hour period was nauseated after the second dose and vomited after the third dose. In studies designed to examine the potential for side effects, intentional ingestion of up to 60 mg of loperamide hydrochloride in a single dose to healthy subjects resulted in no significant adverse effects.
-
DOSAGE AND ADMINISTRATION
(1 capsule = 2 mg)
Patients should receive appropriate fluid and electrolyte replacement as needed.Acute Diarrhea
Adults: The recommended initial dose is 4mg (two capsules) followed by 2 mg (one capsule) after each unformed stool. Daily dose should not exceed 16mg (eight capsules). Clinical improvement is usually observed within 48 hours.
Children: In children 2 to 5 years of age (20 kg or less), the non-prescription liquid formulation (IMODIUM® A-D 1 mg/7.5 mL) should be used; for ages 6 to 12, either IMODIUM® Capsules or IMODIUM® A-D Liquid may be used. For children 2 to 12 years of age, the following schedule for capsules or liquid will usually fulfill initial dosage requirements:
Chronic Diarrhea
Children: Although IMODIUM® has been studied in a limited number of children with chronic diarrhea; the therapeutic dose for the treatment of chronic diarrhea in a pediatric population has not been established.
Adults: The recommended initial dose is 4 mg (two capsules) followed by 2 mg (one capsule) after each unformed stool until diarrhea is controlled, after which the dosage of IMODIUM® should be reduced to meet individual requirements. When the optimal daily dosage has been established, this amount may then be administered as a single dose or in divided doses.
The average daily maintenance dosage in clinical trials was 4 to 8 mg (two to four capsules). A dosage of 16 mg (eight capsules) was rarely exceeded. If clinical improvement is not observed after treatment with 16 mg per day for at least 10 days, symptoms are unlikely to be controlled by further administration. IMODIUM® administration may be continued if diarrhea cannot be adequately controlled with diet or specific treatment.
Children under 2 Years
The use of IMODIUM in children under 2 years is not recommended. There have been rare reports of paralytic ileus associated with abdominal distention. Most of these reports occurred in the setting of acute dysentery, overdose, and with very young children less than two years of age.
Elderly
No formal pharmacokinetic studies were conducted in elderly subjects. However, there were no major differences reported in the drug disposition in elderly patients with diarrhea relative to young patients. No dosage adjustment is required in the elderly.
Renal Impairment
No pharmacokinetic data are available in patients with renal impairment. Since the metabolites and the unchanged drug are mainly excreted in the feces, no dosage adjustment is required for patients with renal impairment (see PRECAUTIONS section).
Hepatic Impairment
Although no pharmacokinetic data are available in patients with hepatic impairment, IMODIUM should be used with caution in such patients because of reduced first pass metabolism. (see Precautions).
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
IMODIUM
loperamide hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50458-400 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Loperamide Hydrochloride (UNII: 77TI35393C) (Loperamide - UNII:6X9OC3H4II) 2 mg Inactive Ingredients Ingredient Name Strength Lactose (UNII: J2B2A4N98G) Cornstarch () Talc (UNII: 7SEV7J4R1U) Magnesium stearate (UNII: 70097M6I30) FD&C Yellow No. 6 () Product Characteristics Color GREEN (Dark green and light green) Score no score Shape CAPSULE (CAPSULE) Size 14mm Flavor Imprint Code JANSSEN;IMODIUM Contains Coating false Symbol false Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50458-400-10 100 in 1 BOTTLE Labeler - McNeil Consumer Healthcare
