Label: 4287 FIRST AID KIT kit
- NDC Code(s): 0498-0100-02, 0498-0143-04, 0498-0750-36, 0498-4287-01
- Packager: Honeywell Safety Products USA, INC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Eyewash Active ingredient
- Eyewash Purpose
- Eyewash Uses
-
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyewash Directions
- Eyewash Inactive ingredients
- Eyewash Questions
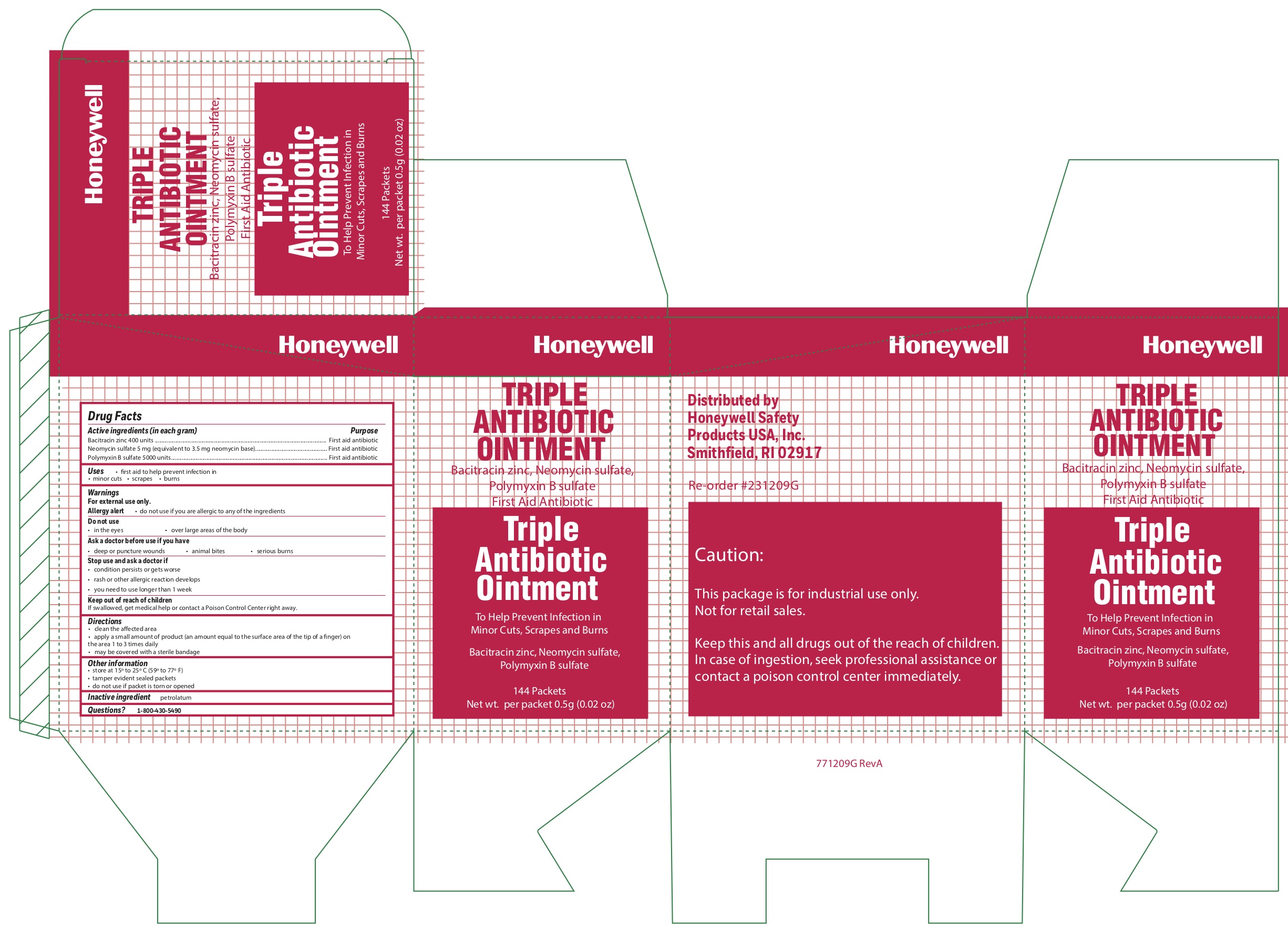
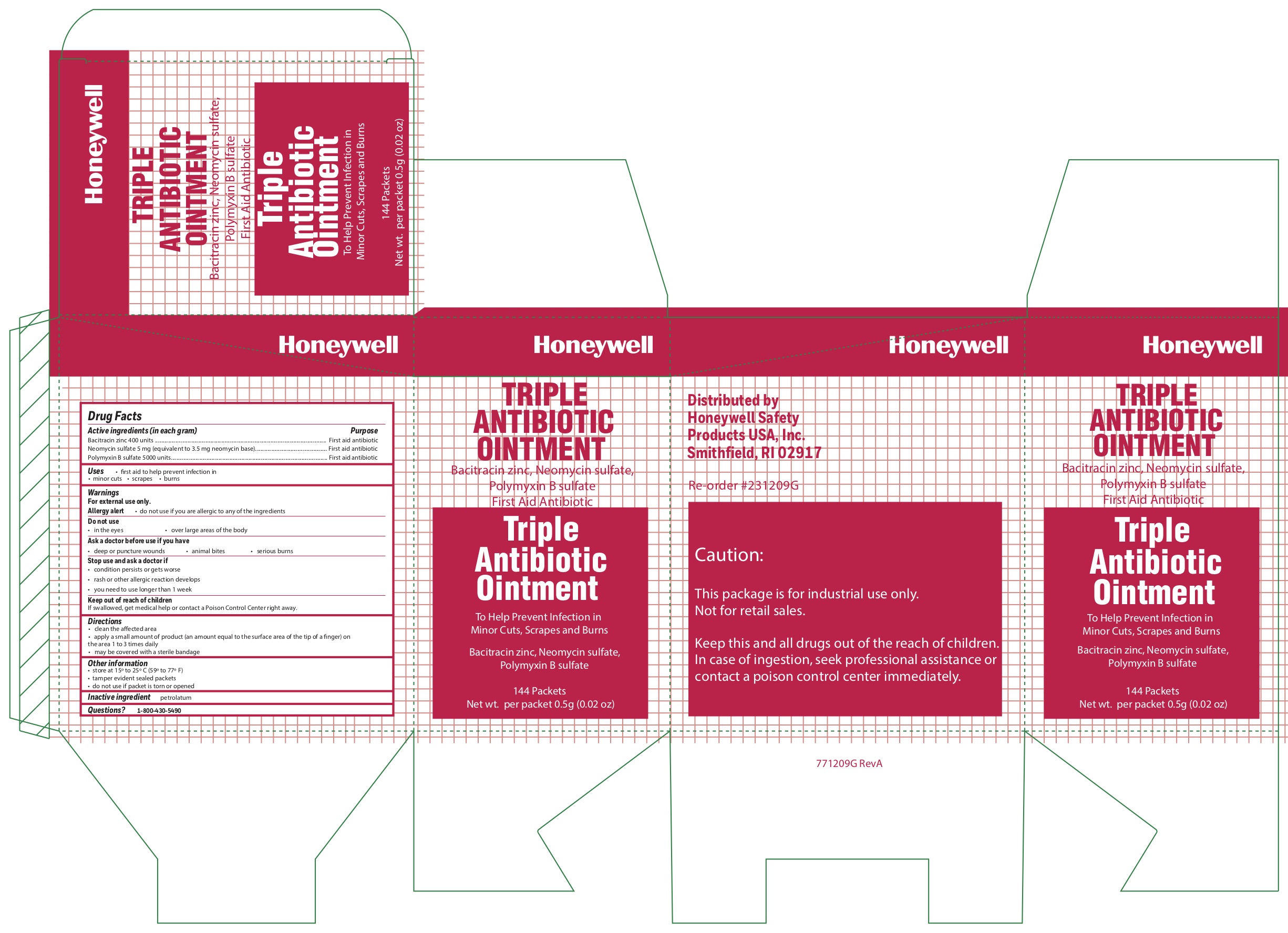
- Triple Active ingredients
- Triple Purpose
- Triple Uses
- Triple Warnings
- Triple Directions
- Triple Other information
- Triple Inactive ingredient
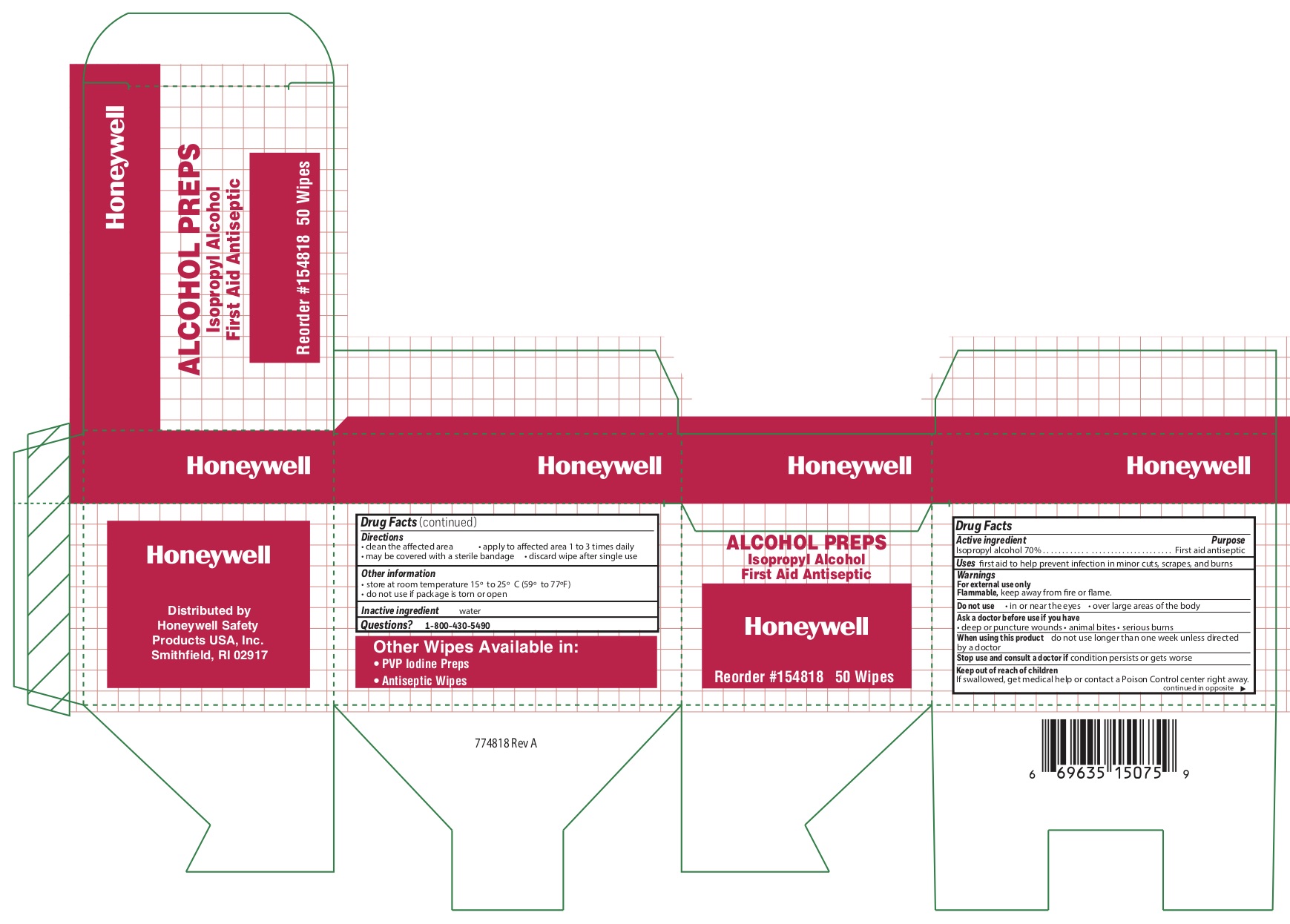
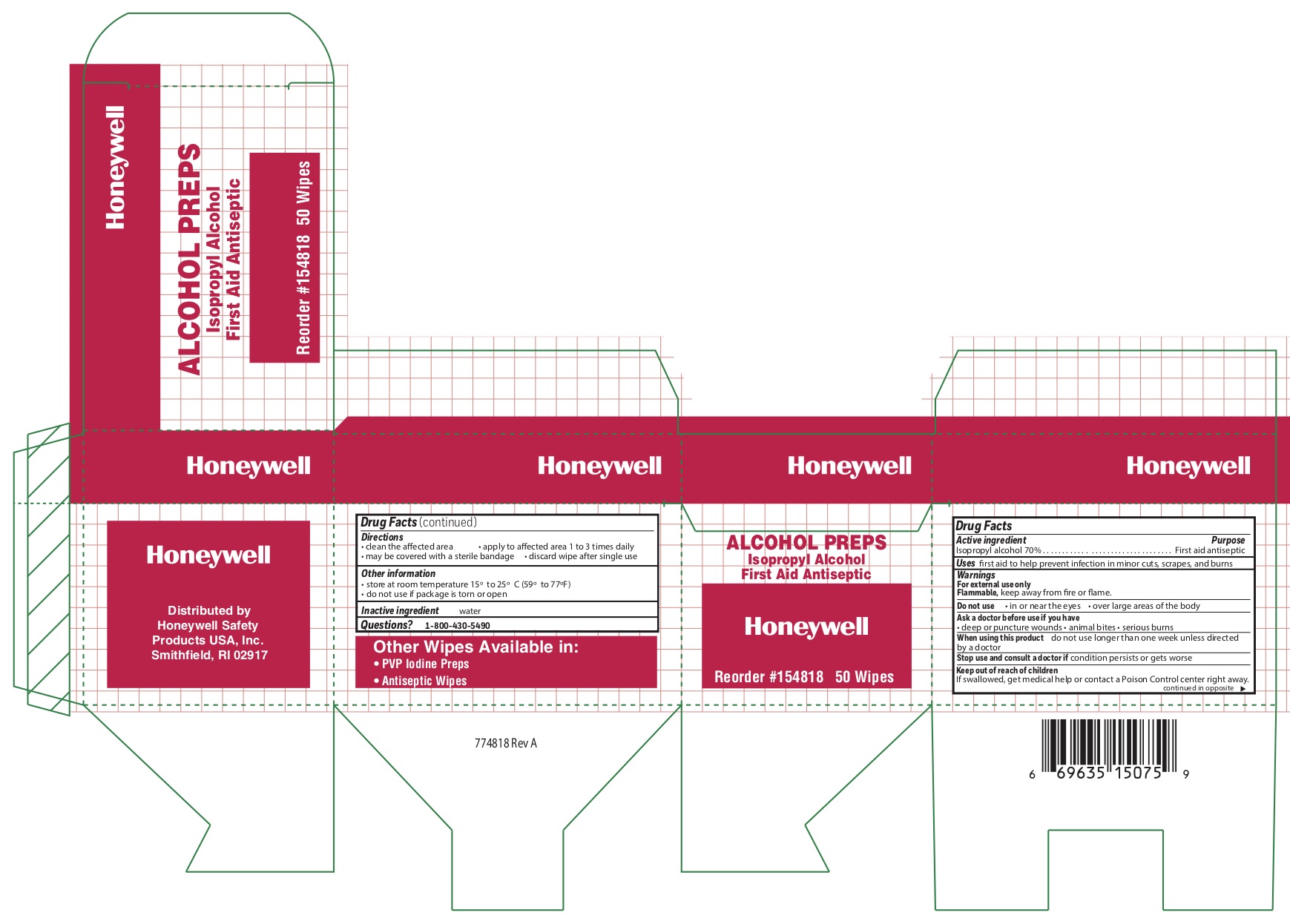
- Alcohol Active ingredient
- Alcohol Purpose
- Alcohol Uses
- Alcohol Warnings
- Alcohol Directions
- Alcohol Other information
- Alcohol Inactive ingredient
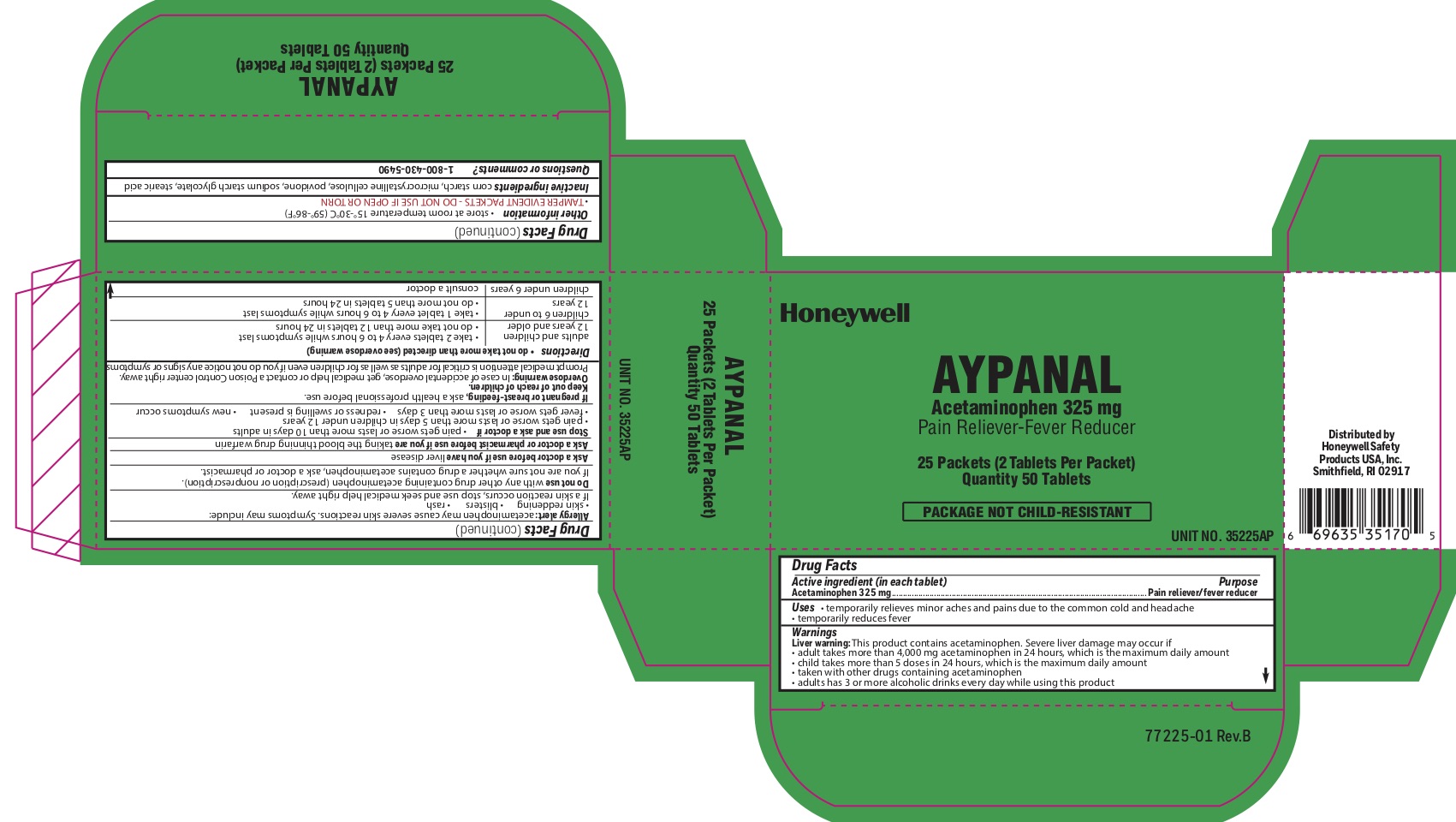
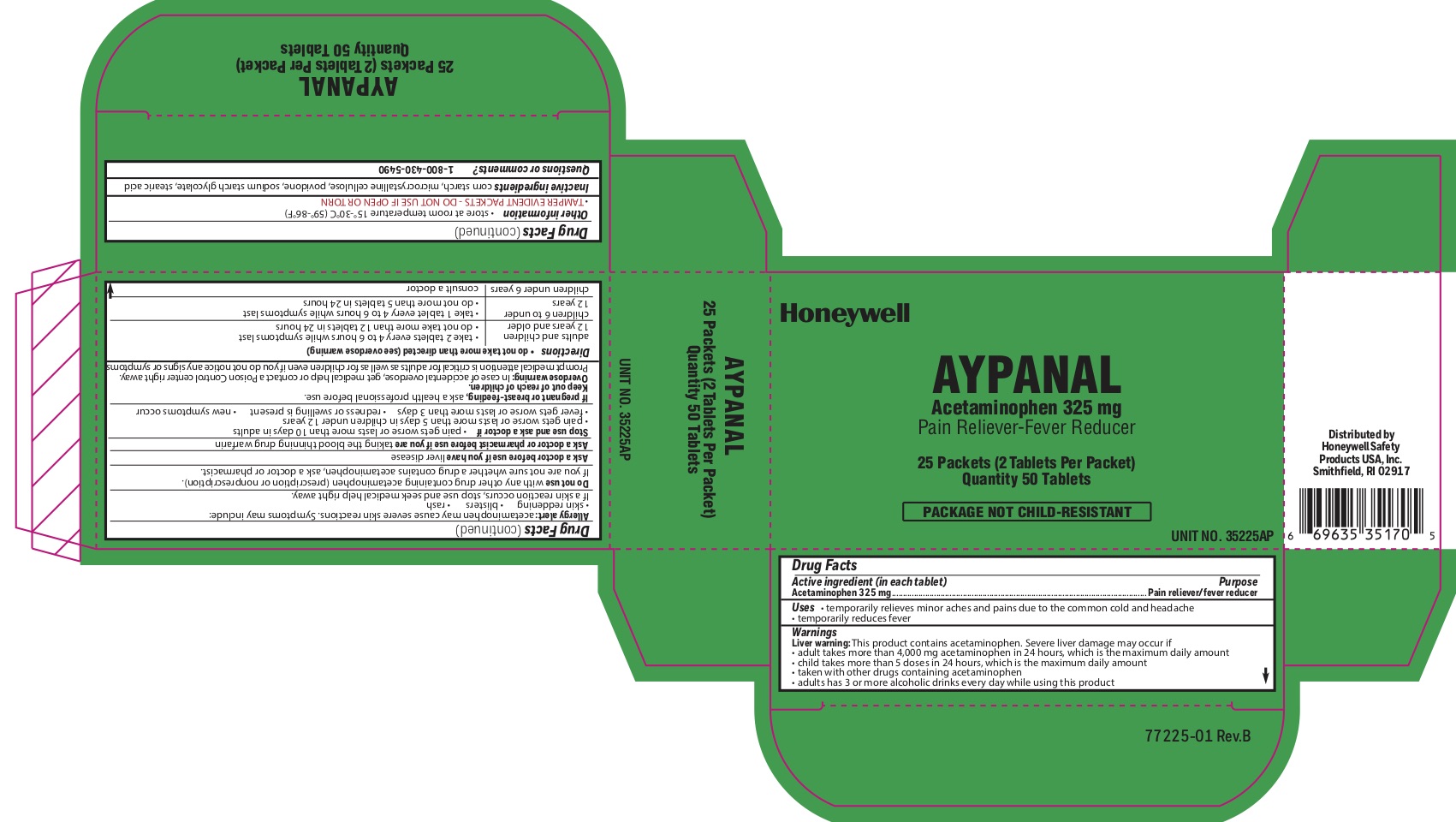
- Aypanal Active ingredient
- Aypanal Purpose
- Aypanal Uses
-
Aypanal
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount.
- with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
Keep out of reach of children.
Keep out of reach of children.
Overdose warning: In case ofl overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
- Aypanal Directions
- Aypanal Other information
- Aypanal Inactive igredients
- Aypanal Questions or Comments
-
4287
SF00003256 KIT CONTENTS
1 1X3 PLASTIC 100/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 SWIFT KNUCKLE 40/BX
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 GZE PADS STERILE 2"X 2" 25'S
1 ALCOHOL WIPES 50'S
1 AYPANAL NON-ASP IND 2/ENV 100
1 TRIPLE BIOTIC .5 GRAM PKT 20
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 F A KIT EMPTY BLANK 140
1 POCKET INSERT RED #140 KIT 2R
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 COLD PACK 5"X 9" BULK
- Eyewash Principal Display Panel
- Triple Principal Display Panel
- Alcohol Principal Display Panel
- Aypanal Principal Display Panel
- 4287 Kit Label SF000003256
-
INGREDIENTS AND APPEARANCE
4287 FIRST AID KIT
4287 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4287 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4287-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 118 mL Part 2 20 PACKET 10 g Part 3 50 POUCH 20 mL Part 4 50 PACKET 100 Part 1 of 4 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC:0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0100-02 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 12/18/2018 Part 2 of 4 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC:0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0750-36 0.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/19/2018 Part 3 of 4 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC:0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 4 of 4 AYPANAL NON-ASPIRIN
acetaminophen tabletProduct Information Item Code (Source) NDC:0498-2001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) Product Characteristics Color white Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code circle;U Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/10/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (118768815)