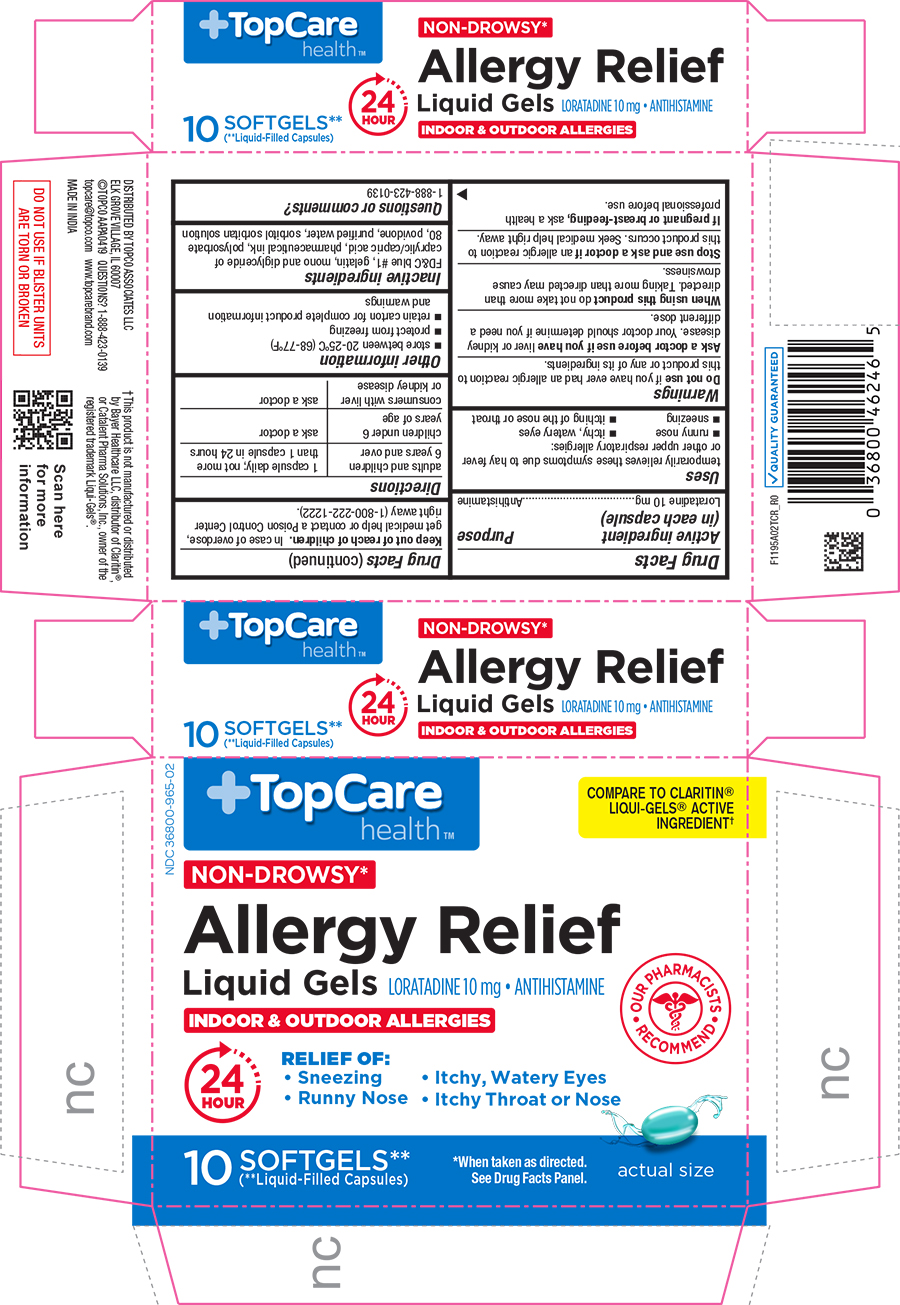
ALLERGY RELIEF- loratadine capsule, liquid filled
TopCare
----------
TCR - 1195A - 2019-1007
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies
• runny nose
• sneezing
• itchy, water eyes
• itching of the nose or throat
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Directions
| adults and children 6 years and over | 1 capsule daily; not more than 1 capsule in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor
|
Other information
• Store between 20-25˚C (68-77˚F)
• protect from freezing
• retain carton for complete information and warnings
Inactive ingredients
FD&C Blue no. 1, gelatine, mono anddiglyceride of caprilic/capric acid, pharmaceutical ink, polysorbate 80,povidone, purified water, sorbitol, sorbitan solution.
PRINCIPAL DISPLAY PANEL
NDC 36800-965-02
TopCare health
COMPARE TO CLARITIN(R) LIQUI-GELS(R) ACTIVE INGREDIENT
NON-DROWSY*
Allergy Relief
Liquid Gels
LORATADINE 10 mg - ANTIHISTAMINE
INDOOR & OUTDOOR ALLERGIES
OUR PHARMACISTS RECOMMEND
24 HOUR
RELIEF OF:
Sneezing
Itchy, Watery Eyes
Runny Nose
Itchy Throat or Nose
10 SOFTGELS** (**Liquid-Filled Capsules)
*When taken as directed. See Drug Facts Panel.
actual size
| ALLERGY RELIEF
loratadine capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - TopCare (006935977) |