Label: 4270 FIRST AID KIT kit
4401 FIRST AID KIT kit
-
NDC Code(s):
0498-0121-00,
0498-0733-00,
0498-3334-00,
0498-4270-01, view more0498-4401-01
- Packager: Honeywell Safety Products USA, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Ammonia Inhalent Active ingredient (in each ampule)
- Ammonia Inhalent Purpose
- Ammonia Inhalent Uses
- Ammonia Inhalent Warnings
- Ammonia Inhalent Directions
- Ammonia Inhalent Other information
- Ammonia Inhalent Inactive ingredients
- Ammonia Inhalent Questions
- PVP Active Ingredient
- PVP Purpose
- PVP Uses
- PVP Warnings
- Keep out of reach of children.
- PVP Directions
- PVP Other informatiion
- PVP Inactive ingredient
- PVP Questions
- Sting Relief Active ingredient (in each wipe)
- Sting Relief Purpose
- Sting Relief Uses
- Sting Relief Warnings
- Stig Relief Directions
- Sting Relief Inactive ingredients
- Questions or Comments?
-
4270
SF00001090 Kit Contents
1 1 X 3 PLASTIC 50/BOX
1 WOVEN 2" X 3" 25/BOX
1 KNUCKLE BAND 8 PER
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
2 INSTANT COLD PACK 4" X 6"
1 FINGERTIP BANDAGE, 10 PER
1 PVP IODINE WIPES 10 PER
2 ADHES TAPE W/P 1"X 2 1/2 YD
2 GAUZE BANDAGE 2"X2 YDS STRETCH GZ
1 FIRST AID GUIDE ASHI
1 GZE PADS STERILE 3"X 3" 10'S
1 CPR FILTERSHIELD 77-100
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 180 EMPTY BLANK NO LOGO
1 POCKET INSERT RED #180 KIT 4R
2 BANDAGE COMP 4" W/TELFA PAD 1
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
3 PR LRG NITRILE GLVES ZIP BAG
1 WATER-JEL BURN DRESSING 4 X 4
1 NOX A STING WIPES 10
-
4401
SF00001103 kit contents
1 1 X 3 PLASTIC 50/BOX
1 WOVEN 2" X 3" 25/BOX
1 KNUCKLE BAND 8 PER
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
2 INSTANT COLD PACK 4" X 6"
1 FINGERTIP BANDAGE, 10 PER
1 PVP IODINE WIPES 10 PER
2 ADHES TAPE W/P 1"X 2 1/2 YD
2 GAUZE BANDAGE 2"X2 YDS STRETCH GZ
1 FIRST AID GUIDE ASHI
1 GZE PADS STERILE 3"X 3" 10'S
1 CPR FILTERSHIELD 77-100
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
2 BANDAGE COMP 4" W/TELFA PAD 1
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
3 PR LRG NITRILE GLVES ZIP BAG
1 WATER-JEL BURN DRESSING 4 X 4
1 POLYBAG 14" X 20" '3 MIL'
1 STING relief WIPES 10
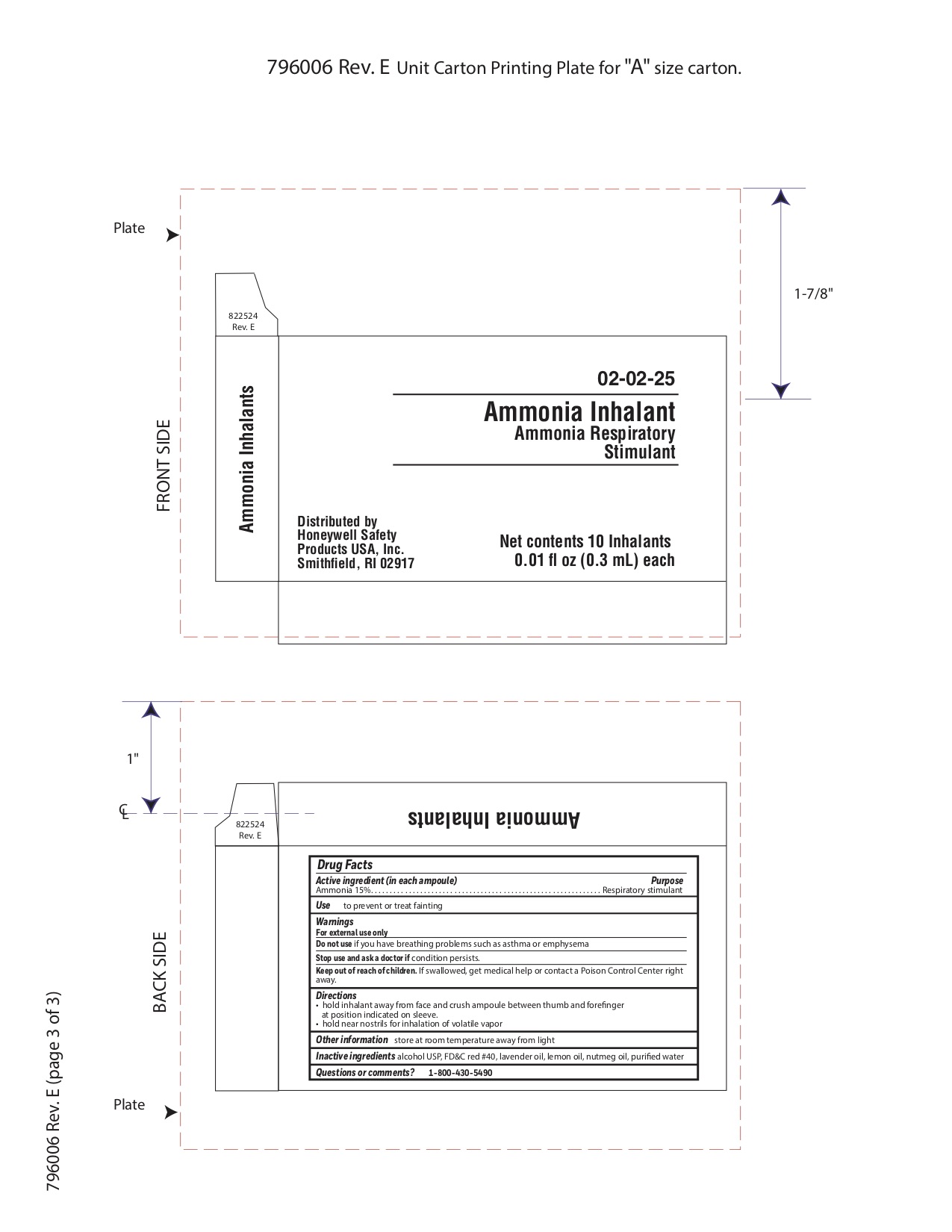
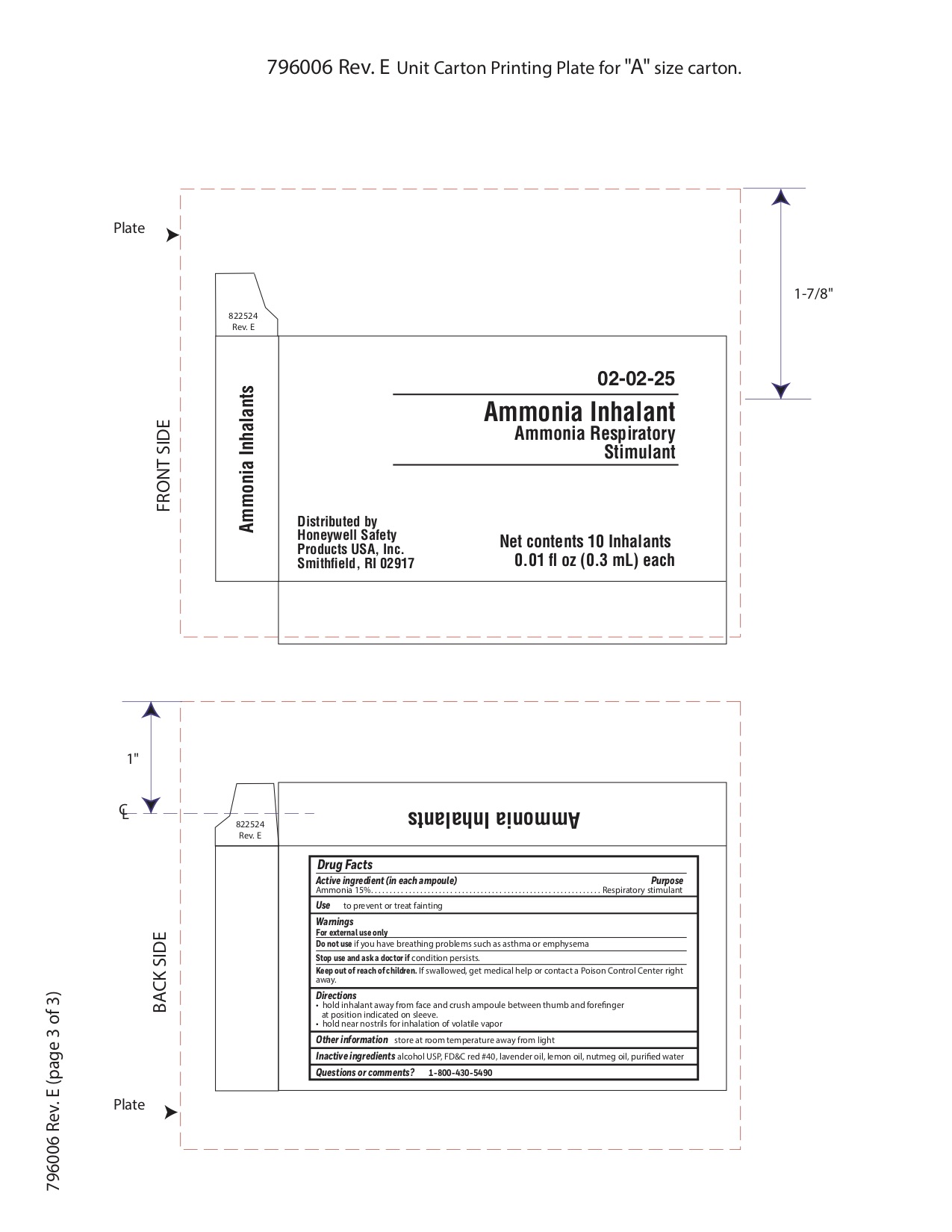
- Ammonia Inahalent Principal Display Panel
- PVP Principal Display Panel
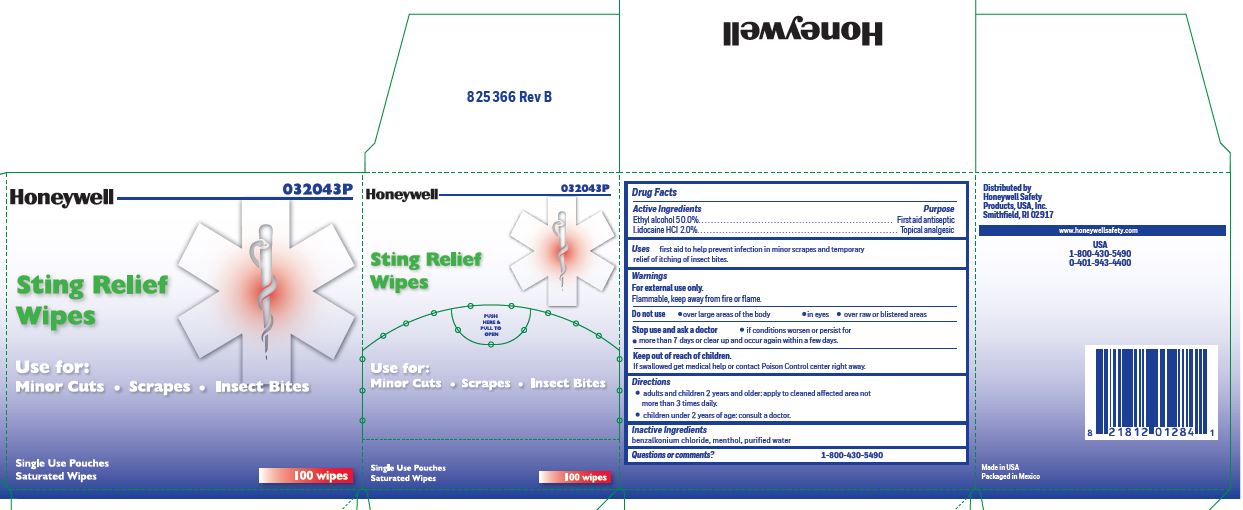
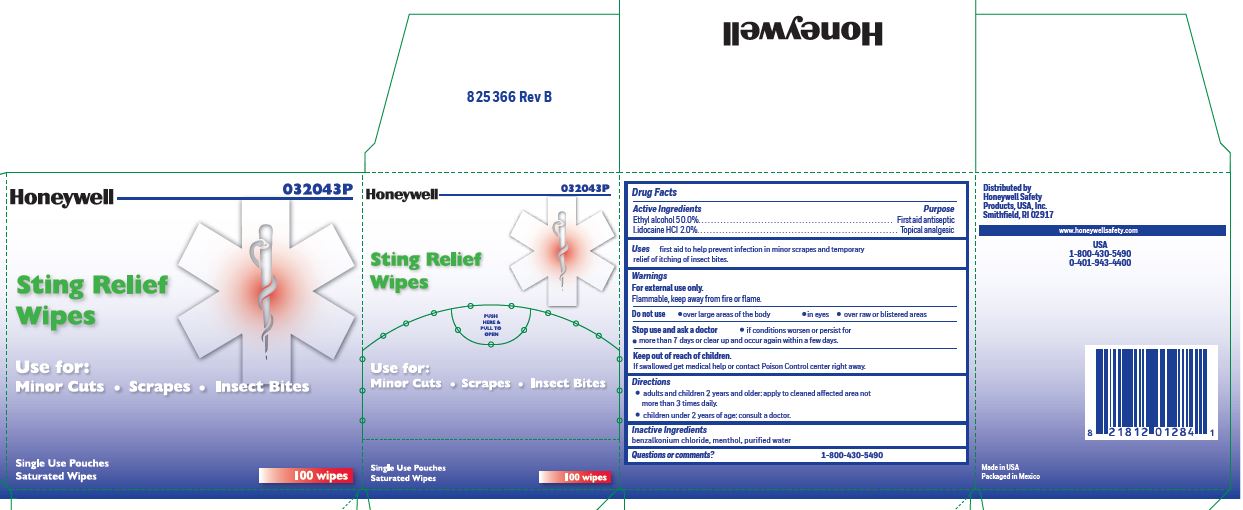
- Sting Relief Principal Display Panel
- 4270 Kit Label SF00001090
- 4401 Kit Label SF00001103
-
INGREDIENTS AND APPEARANCE
4270 FIRST AID KIT
4270 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4270 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4270-01 1 in 1 KIT; Type 0: Not a Combination Product 03/14/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 AMPULE 3 mL Part 2 10 POUCH 3 mL Part 3 10 POUCH 4 mL Part 1 of 3 AMMONIA INHALENT
ammonia inhalent inhalantProduct Information Item Code (Source) NDC:0498-3334 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.045 g in 0.3 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-3334-00 0.3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 2 of 3 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC:0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 3 of 3 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC:0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0733-00 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/14/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/14/2019 01/17/2020 4401 FIRST AID KIT
4401 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4401 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4401-01 1 in 1 KIT; Type 0: Not a Combination Product 03/14/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 AMPULE 3 mL Part 2 10 POUCH 3 mL Part 3 10 POUCH 4 mL Part 1 of 3 AMMONIA INHALENT
ammonia inhalent inhalantProduct Information Item Code (Source) NDC:0498-3334 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.045 g in 0.3 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-3334-00 0.3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 12/01/2022 Part 2 of 3 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC:0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 3 of 3 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC:0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0733-00 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/14/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/14/2019 Labeler - Honeywell Safety Products USA, INC (118768815)