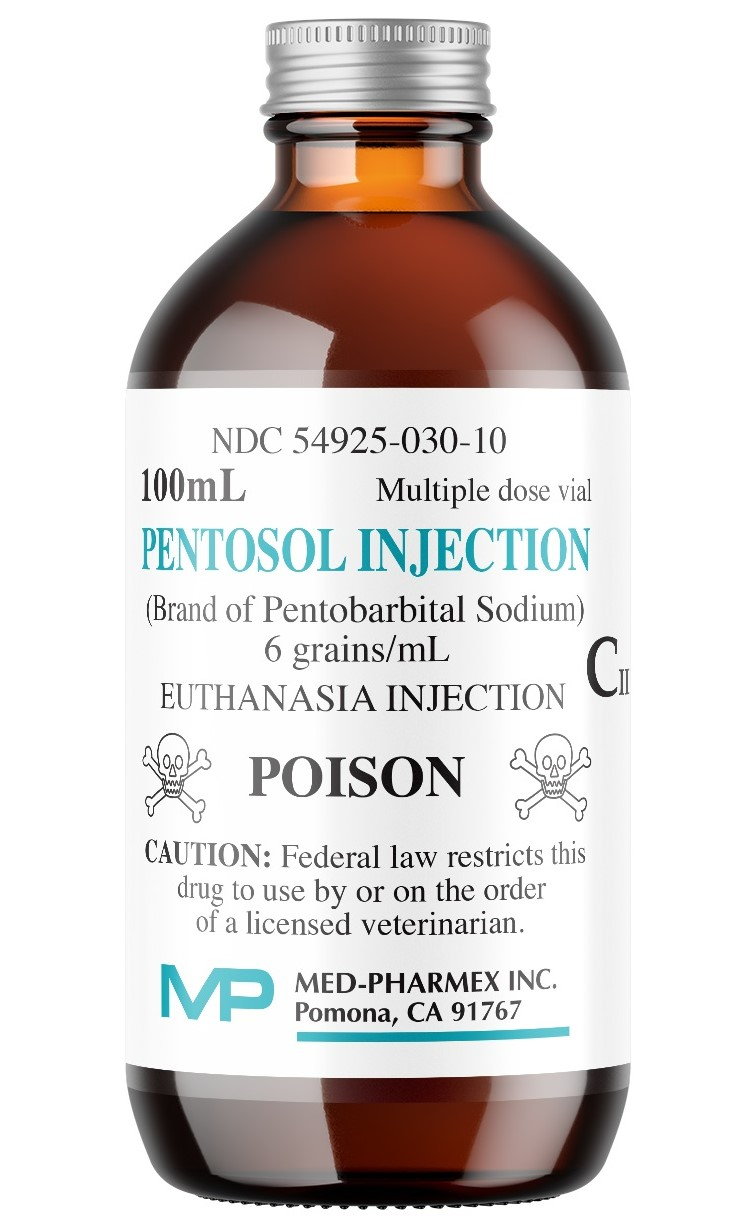
Label: PENTOSOL- euthanasia injection, solution
- NDC Code(s): 54925-030-25
- Packager: Med-Pharmex, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 12, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
DOSAGE AND ADMINISTRATION:
Mulitple Dose Vial
Dosage: For intravenous (preferred), intracardial, intrapleural, or intraperitoneal injection.
Small Animals: Inject rapidly 1 mL per 10 lbs. of bady weight. Minimum dose 1 mL.
Horses and other Large Animals: Inject rapidly 1 mL per 10 lbs. of obdy weight, to a maximum of 100 mL.
Each mL contains:
Pentobarbital sodium ..... 6 gr.
Isopropyl alcohol .......... 10%
Propylene glycol ........... 18%
Benzyl alcohol .............. 2.0%
Purified water ............... qs
Green dye to establish a distinctive color.
TAKE TIME - OBSERVE LABEL DIRECTIONS
- WARNING:
- ENVIRONMENTAL HAZARD:
-
ADVERSE REACTIONS
To report suspected adverse drug events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Med-Pharmex at (909) 593-7875. For additional information about adverse drug experience reporting for animals, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae.
Manufactured by:
Med-Pharmex, Inc.
2727 Thompson Creek Road
Pomona, CA 91767Rev.: 01/2023
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PENTOSOL
euthanasia injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54925-030 Route of Administration INTRAVENOUS, INTRACARDIAC, INTRAPLEURAL, INTRAPERITONEAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PENTOBARBITAL SODIUM (UNII: NJJ0475N0S) (PENTOBARBITAL - UNII:I4744080IR) PENTOBARBITAL SODIUM 390 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) 0.10 mL in 1 mL PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 0.180 mL in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 0.02 mL in 1 mL WATER (UNII: 059QF0KO0R) 0.01 mL in 1 mL Product Characteristics Color green (Green dye to establish a distinctive color.) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54925-030-25 100 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/03/2000 Labeler - Med-Pharmex, Inc. (025353699) Registrant - Med-Pharmex, Inc. (025353699) Establishment Name Address ID/FEI Business Operations Med-Pharmex, Inc. 025353699 manufacture Establishment Name Address ID/FEI Business Operations Siegfried USA, LLC 001213784 api manufacture