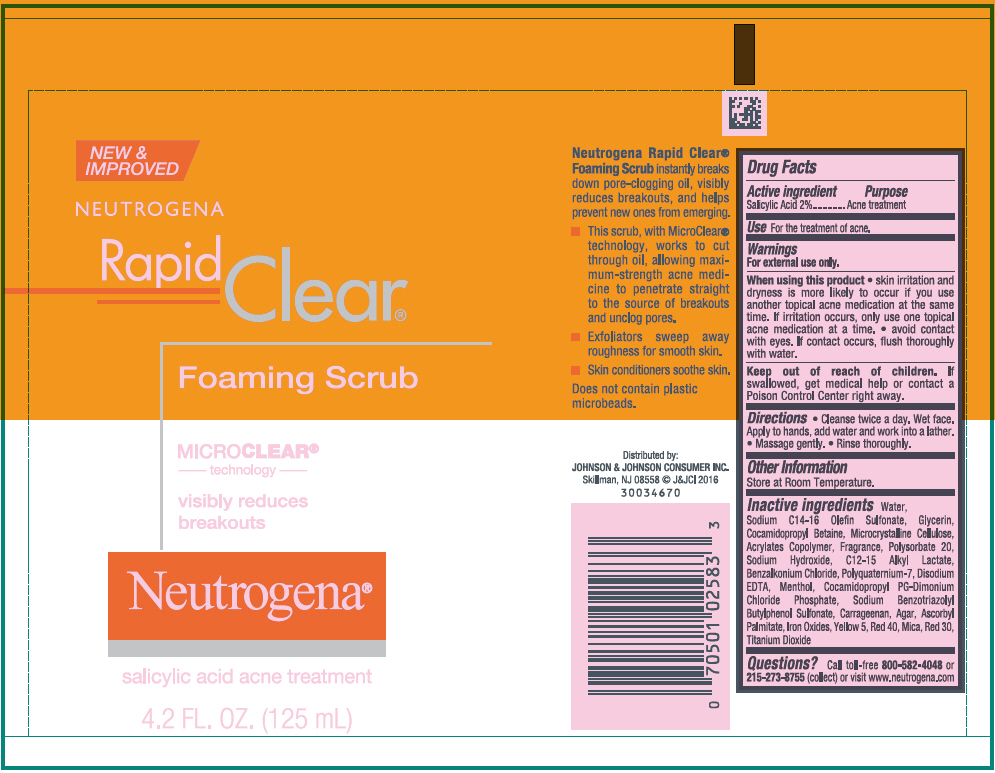
NEUTROGENA RAPID CLEAR FOAMING SCRUB- salicylic acid gel
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
NEUTROGENA Rapid Clear ® Foaming Scrub
Warnings
For external use only.
Directions
- Cleanse twice a day. Wet face. Apply to hands, add water and work into a lather.
- Massage gently.
- Rinse thoroughly.
Inactive ingredients
Water, Sodium C14-16 Olefin Sulfonate, Glycerin, Cocamidopropyl Betaine, Microcrystalline Cellulose, Acrylates Copolymer, Fragrance, Polysorbate 20, Sodium Hydroxide, C12-15 Alkyl Lactate, Benzalkonium Chloride, Polyquaternium-7, Disodium EDTA, Menthol, Cocamidopropyl PG-Dimonium Chloride Phosphate, Sodium Benzotriazolyl Butylphenol Sulfonate, Carrageenan, Agar, Ascorbyl Palmitate, Iron Oxides, Yellow 5, Red 40, Mica, Red 30, Titanium Dioxide
| NEUTROGENA RAPID CLEAR FOAMING SCRUB
salicylic acid gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |