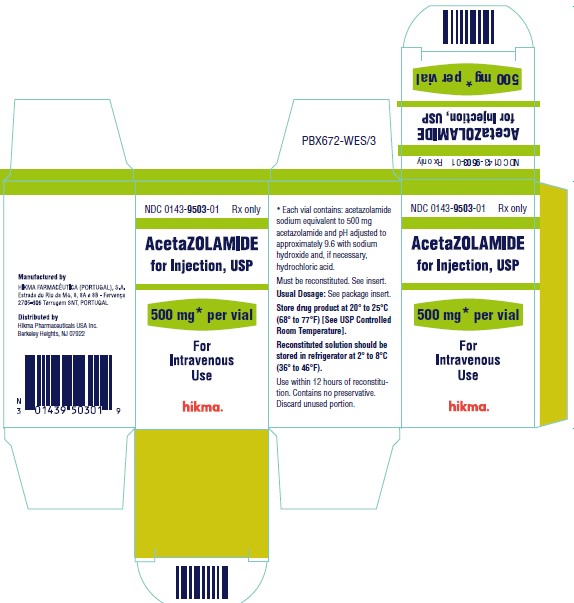
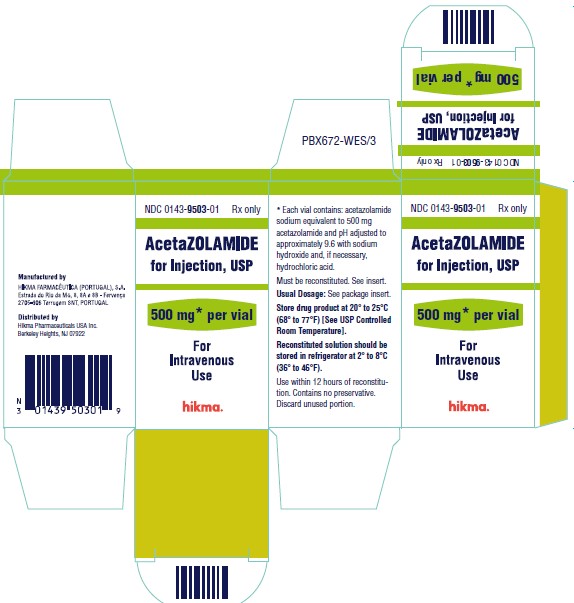
Label: ACETAZOLAMIDE injection, powder, lyophilized, for solution
- NDC Code(s): 0143-9503-01
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Acetazolamide, an inhibitor of the enzyme carbonic anhydrase, is a white to faintly yellowish white crystalline, odorless powder, weakly acidic, very slightly soluble in water and slightly soluble in alcohol. The chemical name for acetazolamide is N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl) acetamide and has the following chemical structure:
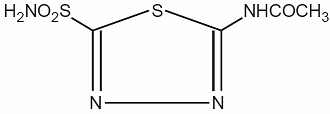
M.W. 222.24 C4H6N4O3S2
Acetazolamide for Injection, USP is available for intravenous use, and is supplied as a sterile powder requiring reconstitution. Each vial contains an amount of acetazolamide sodium equivalent to 500 mg of acetazolamide. The bulk solution is adjusted to pH 9.6 using sodium hydroxide and, if necessary, hydrochloric acid prior to lyophilization.
-
CLINICAL PHARMACOLOGY
Acetazolamide for Injection is a potent carbonic anhydrase inhibitor, effective in the control of fluid secretion (e.g., some types of glaucoma), in the treatment of certain convulsive disorders (e.g., epilepsy) and in the promotion of diuresis in instances of abnormal fluid retention (e.g., cardiac edema).
Acetazolamide for Injection is not a mercurial diuretic. Rather, it is a nonbacteriostatic sulfonamide possessing a chemical structure and pharmacological activity distinctly different from the bacteriostatic sulfonamides.
Acetazolamide for Injection is an enzyme inhibitor that acts specifically on carbonic anhydrase, the enzyme that catalyzes the reversible reaction involving the hydration of carbon dioxide and the dehydration of carbonic acid. In the eye, this inhibitory action of acetazolamide decreases the secretion of aqueous humor and results in a drop in intraocular pressure, a reaction considered desirable in cases of glaucoma and even in certain nonglaucomatous conditions. Evidence seems to indicate that Acetazolamide for Injection has utility as an adjuvant in the treatment of certain dysfunctions of the central nervous system (e.g., epilepsy). Inhibition of carbonic anhydrase in this area appears to retard abnormal, paroxysmal, excessive discharge from central nervous system neurons. The diuretic effect of Acetazolamide for Injection is due to its action in the kidney on the reversible reaction involving hydration of carbon dioxide and dehydration of carbonic acid.
The result is renal loss of HCO3 ion, which carries out sodium, water, and potassium. Alkalinization of the urine and promotion of diuresis are thus affected. Alteration in ammonia metabolism occurs due to increased reabsorption of ammonia by the renal tubules as a result of urinary alkalinization.
-
INDICATIONS AND USAGE
For adjunctive treatment of: edema due to congestive heart failure; drug-induced edema; centrencephalic epilepsies (petit mal, unlocalized seizures); chronic simple (open-angle) glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where delay of surgery is desired in order to lower intraocular pressure.
-
CONTRAINDICATIONS
Hypersensitivity to acetazolamide or any excipients in the formulation. Since acetazolamide is a sulfonamide derivative, cross sensitivity between acetazolamide, sulfonamides and other sulfonamide derivatives is possible.
Acetazolamide for Injection therapy is contraindicated in situations in which sodium and/or potassium blood serum levels are depressed, in cases of marked kidney and liver disease or dysfunction, in suprarenal gland failure, and in hyperchloremic acidosis. It is contraindicated in patients with cirrhosis because of the risk of development of hepatic encephalopathy.
Long-term administration of Acetazolamide for Injection is contraindicated in patients with chronic noncongestive angle-closure glaucoma since it may permit organic closure of the angle to occur while the worsening glaucoma is masked by lowered intraocular pressure.
-
WARNINGS
Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias, and anaphylaxis. Sensitizations may recur when a sulfonamide is readministered irrespective of the route of administration. If signs of hypersensitivity or other serious reactions occur, discontinue use of this drug.
Caution is advised for patients receiving concomitant high-dose aspirin and Acetazolamide for Injection, as anorexia, tachypnea, lethargy, metabolic acidosis, coma, and death have been reported.
-
PRECAUTIONS
General
Increasing the dose does not increase the diuresis and may increase the incidence of drowsiness and/or paresthesia. Increasing the dose often results in a decrease in diuresis. Under certain circumstances, however, very large doses have been given in conjunction with other diuretics in order to secure diuresis in complete refractory failure.
Information for Patients
Adverse reactions common to all sulfonamide derivatives may occur: anaphylaxis, fever, rash (including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), crystalluria, renal calculus, bone marrow depression, thrombocytopenic purpura, hemolytic anemia, leukopenia, pancytopenia and agranulocytosis. Caution is advised for early detection of such reactions and the drug should be discontinued and appropriate therapy instituted.
In patients with pulmonary obstruction or emphysema where alveolar ventilation may be impaired, Acetazolamide for Injection which may precipitate or aggravate acidosis, should be used with caution.
Caution is advised for patients receiving concomitant high-dose aspirin and Acetazolamide for Injection, as anorexia, tachypnea, lethargy, metabolic acidosis, coma, and death have been reported (see WARNINGS).
Both increases and decreases in blood glucose levels have been described in patients treated with acetazolamide. This should be taken into consideration in patients with impaired glucose tolerance or diabetes mellitus.
Acetazolamide treatment may cause electrolyte imbalances, including hyponatremia and hypokalemia, as well as metabolic acidosis. Therefore, periodic monitoring of serum electrolytes is recommended. Particular caution is recommended in patients with conditions that are associated with, or predispose a patient to, electrolyte and acid/base imbalances, such as patients with impaired renal function (including elderly patients; see PRECAUTIONS, Geriatric Use), patients with diabetes mellitus, and patients with impaired alveolar ventilation.
Some adverse reactions to acetazolamide, such as drowsiness, fatigue, and myopia, may impair the ability to drive and operate machinery.
Laboratory Tests
To monitor for hematologic reactions common to all sulfonamides, it is recommended that a baseline CBC and platelet count be obtained on patients prior to initiating Acetazolamide for Injection therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. Periodic monitoring of serum electrolytes is recommended.
Drug Interactions
Aspirin - See WARNINGS.
Acetazolamide for Injection modifies phenytoin metabolism with increased serum levels of phenytoin. This may increase or enhance the occurrence of osteomalacia in some patients receiving chronic phenytoin therapy. Caution is advised in patients receiving chronic concomitant therapy.
By decreasing the gastrointestinal absorption of primidone, Acetazolamide for Injection may decrease serum concentrations of primidone and its metabolites, with a consequent possible decrease in anticonvulsant effect. Caution is advised when beginning, discontinuing, or changing the dose of Acetazolamide for Injection in patients receiving primidone.
Because of possible additive effects with other carbonic anhydrase inhibitors, concomitant use is not advisable.
Acetazolamide may increase the effects of other folic acid antagonists.
Acetazolamide may increase or decrease blood glucose levels. Consideration should be taken in patients being treated with antidiabetic agents.
Acetazolamide decreases urinary excretion of amphetamine and may enhance the magnitude and duration of their effect.
Acetazolamide reduces urinary excretion of quinidine and may enhance its effect. Acetazolamide may prevent the urinary antiseptic effect of methenamine.
Acetazolamide increases lithium excretion and the lithium may be decreased.
Acetazolamide and sodium bicarbonate used concurrently increases the risk of renal calculus formation. Acetazolamide may elevate cyclosporine levels.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of Acetazolamide for Injection have not been conducted. In a bacterial mutagenicity assay, Acetazolamide for Injection was not mutagenic when evaluated with and without metabolic activation.
The drug had no effect on fertility when administered in the diet to male and female rats at a daily intake of up to 4 times the recommended human dose of 1000 mg in a 50 kg individual.
Pregnancy:
Teratogenic Effects
Acetazolamide, administered orally or parenterally, has been shown to be teratogenic (defects of the limbs) in mice, rats, hamsters and rabbits. There are no adequate and well-controlled studies in pregnant women. Acetazolamide should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from Acetazolamide for Injection, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother. Acetazolamide should only be used by nursing women if the potential benefit justifies the potential risk to the child.
Pediatric Use
The safety and effectiveness of Acetazolamide for Injection in pediatric patients have not been established.
Growth retardation has been reported in children receiving long-term therapy, believed secondary to chronic acidosis.
Geriatric Use
Metabolic acidosis, which can be severe, may occur in the elderly with reduced renal function.
Clinical studies of acetazolamide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Body as a whole: Headache, malaise, fatigue, fever, pain at injection site, flushing, growth retardation in children, flaccid paralysis, anaphylaxis
Digestive: Gastrointestinal disturbances such as nausea, vomiting, diarrhea
Hematological/Lymphatic: Blood dyscrasias such as aplastic anemia, agranulocytosis, leukopenia, thrombocytopenia, thrombocytopenic purpura, melena
Hepato-biliary disorders: Abnormal liver function, cholestatic jaundice, hepatic insufficiency, fulminant hepatic necrosis
Metabolic/Nutritional: Metabolic acidosis, electrolyte imbalance, including hypokalemia, hyponatremia, osteomalacia with long-term phenytoin therapy, loss of appetite, taste alteration, hyper/hypoglycemia
Nervous: Drowsiness, paraesthesia (including numbness and tingling of extremities and face), depression, excitement, ataxia, confusion, convulsions, dizziness
Skin: Allergic skin reactions including urticaria, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis
Special senses: Hearing disturbances, tinnitus, transient myopia. Transient myopia is the result of forward movement of the ciliary body leading to a narrowing of the angle.
Urogenital: Crystalluria, increased risk of nephrolithiasis with long-term therapy, hematuria, glycosuria, renal failure, polyuria
-
OVERDOSAGE
No specific antidote is known. Treatment should be symptomatic and supportive.
Electrolyte imbalance, development of an acidotic state, and central nervous effects might be expected to occur. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored.
Supportive measures are required to restore electrolyte and pH balance. The acidotic state can usually be corrected by the administration of bicarbonate.
Despite its high intraerythrocytic distribution and plasma protein binding properties, Acetazolamide for Injection is dialyzable. This may be particularly important in the management of Acetazolamide for Injection overdosage when complicated by the presence of renal failure.
-
DOSAGE AND ADMINISTRATION
Preparation and Storage of Parenteral Solution
Each 500 mg vial containing sterile acetazolamide sodium should be reconstituted with at least 5 mL of Sterile Water for Injection prior to use. Reconstituted solutions retain their physical and chemical properties for 3 days under refrigeration at 2° to 8°C (36° to 46°F), or 12 hours at room temperature 20° to 25°C (68° to 77°F). Contains no preservative. The direct intravenous route of administration is preferred. Intramuscular administration is not recommended.
Glaucoma
Acetazolamide for Injection should be used as an adjunct to the usual therapy. The dosage employed in the treatment of chronic simple (open-angle) glaucoma ranges from 250 mg to 1 g of Acetazolamide for Injection per 24 hours, usually in divided doses for amounts over 250 mg. It has usually been found that a dosage in excess of 1 g per 24 hours does not produce an increased effect. In all cases, the dosage should be adjusted with careful individual attention both to symptomatology and ocular tension. Continuous supervision by a physician is advisable.
In treatment of secondary glaucoma and in the preoperative treatment of some cases of acute congestive (closed-angle) glaucoma, the preferred dosage is 250 mg every four hours, although some cases have responded to 250 mg twice daily on short-term therapy. In some acute cases, it may be more satisfactory to administer an initial dose of 500 mg followed by 125 or 250 mg every four hours depending on the individual case. Intravenous therapy may be used for rapid relief of ocular tension in acute cases. A complementary effect has been noted when Acetazolamide for Injection has been used in conjunction with miotics or mydriatics as the case demanded.
Epilepsy
It is not clearly known whether the beneficial effects observed in epilepsy are due to direct inhibition of carbonic anhydrase in the central nervous system or whether they are due to the slight degree of acidosis produced by the divided dosage. The best results to date have been seen in petit mal in pediatric patients. Good results, however, have been seen in patients, both pediatric patients and adult, in other types of seizures such as grand mal, mixed seizure patterns, myoclonic jerk patterns, etc. The suggested total daily dose is 8 to 30 mg per kg in divided doses. Although some patients respond to a low dose, the optimum range appears to be from 375 to 1000 mg daily. However, some investigators feel that daily doses in excess of 1 g do not produce any better results than a 1 g dose. When Acetazolamide for Injection is given in combination with other anticonvulsants, it is suggested that the starting dose should be 250 mg once daily in addition to the existing medications. This can be increased to levels as indicated above.
The change from other medications to Acetazolamide for Injection should be gradual and in accordance with usual practice in epilepsy therapy.
Congestive Heart Failure
For diuresis in congestive heart failure, the starting dose is usually 250 to 375 mg once daily in the morning (5 mg/kg). If, after an initial response, the patient fails to continue to lose edema fluid, do not increase the dose but allow for kidney recovery by skipping medication for a day.
Acetazolamide for Injection yields best diuretic results when given on alternate days, or for two days alternating with a day of rest.
Failures in therapy may be due to overdosage or too frequent dosage. The use of Acetazolamide for Injection does not eliminate the need for other therapy such as digitalis, bed rest, and salt restriction.
Drug-Induced Edema
Recommended dosage is 250 to 375 mg of Acetazolamide for Injection once a day for one or two days, alternating with a day of rest.
Note: The dosage recommendations for glaucoma and epilepsy differ considerably from those for congestive heart failure, since the first two conditions are not dependent upon carbonic anhydrase inhibition in the kidney which requires intermittent dosage if it is to recover from the inhibitory effect of the therapeutic agent.
Interference with Laboratory Tests
Sulfonamides may give false negative or decreased values for urinary phenolsulfonphthalein and phenol red elimination values for urinary protein, serum non-protein and for serum uric acid.
Acetazolamide may produce an increased level of crystals in the urine.
Acetazolamide interferes with the HPLC method of assay for theophylline. Interference with the theophylline assay by acetazolamide depends on the solvent used in the extraction; acetazolamide may not interfere with other assay methods for theophylline.
-
HOW SUPPLIED
Acetazolamide for Injection, USP sterile intravenous (lyophilized) powder.
NDC 0143-9503-01 500 mg Vial
Store drug product at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Reconstituted solution should be stored in refrigerator at 2° to 8°C (36° to 46°F). Use within 12 hours of reconstitution.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Contains no preservative. Discard unused portion.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
Manufactured by:
HIKMA FARMACÊUTICA (PORTUGAL), S.A.
Estrada do Rio da Mó, 8, 8A e 8B – Fervença – 2705-906 Terrugem SNT, PORTUGAL
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
Revised July 2022
PIN426-WES/3
- VIAL LABEL
- CARTON LABEL
-
INGREDIENTS AND APPEARANCE
ACETAZOLAMIDE
acetazolamide injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0143-9503 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAZOLAMIDE SODIUM (UNII: 429ZT169UH) (ACETAZOLAMIDE - UNII:O3FX965V0I) ACETAZOLAMIDE 500 mg Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0143-9503-01 1 in 1 BOX, UNIT-DOSE 05/01/1996 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040089 05/01/1996 Labeler - Hikma Pharmaceuticals USA Inc. (001230762)