4234 FIRST AID KIT- 4234 first aid
Honeywell Safety Products USA, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-4234: First Aid Kit (Triple, Ammonia Inh, Burn Jel, Hydrogen Peroxide Sp, BZK wipe- 68180SIGMA)
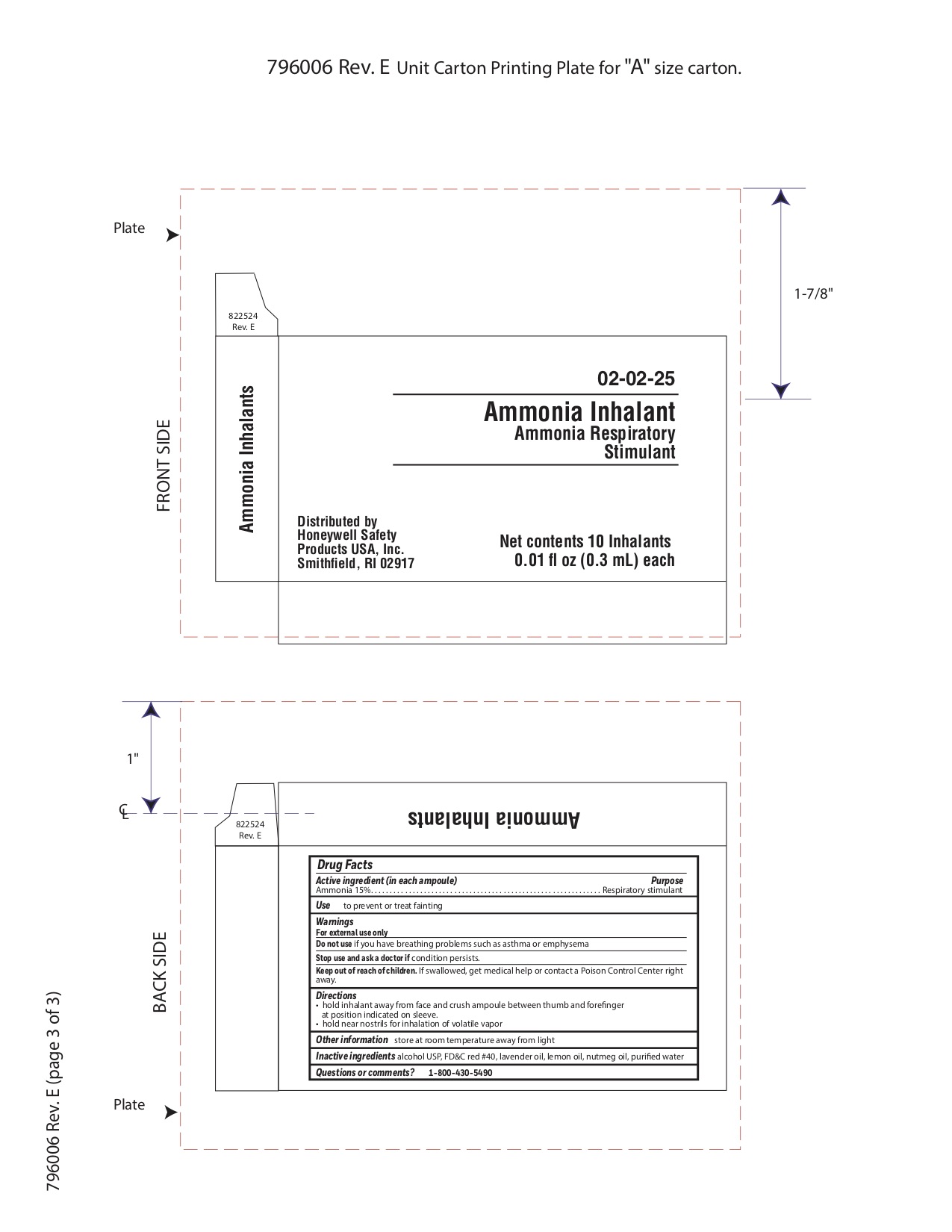
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
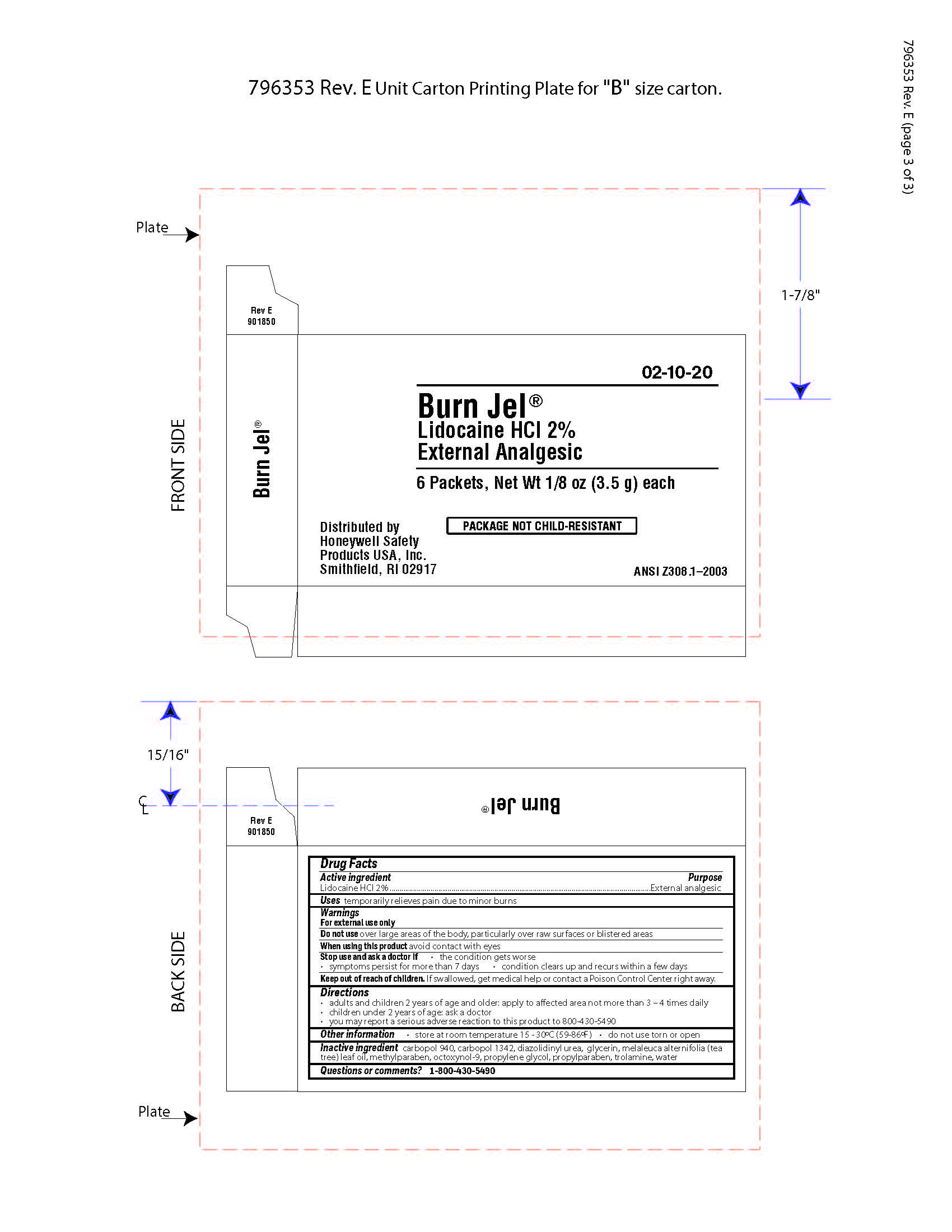
Burn Jel
Warnings
For external use only
Burn JEl
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
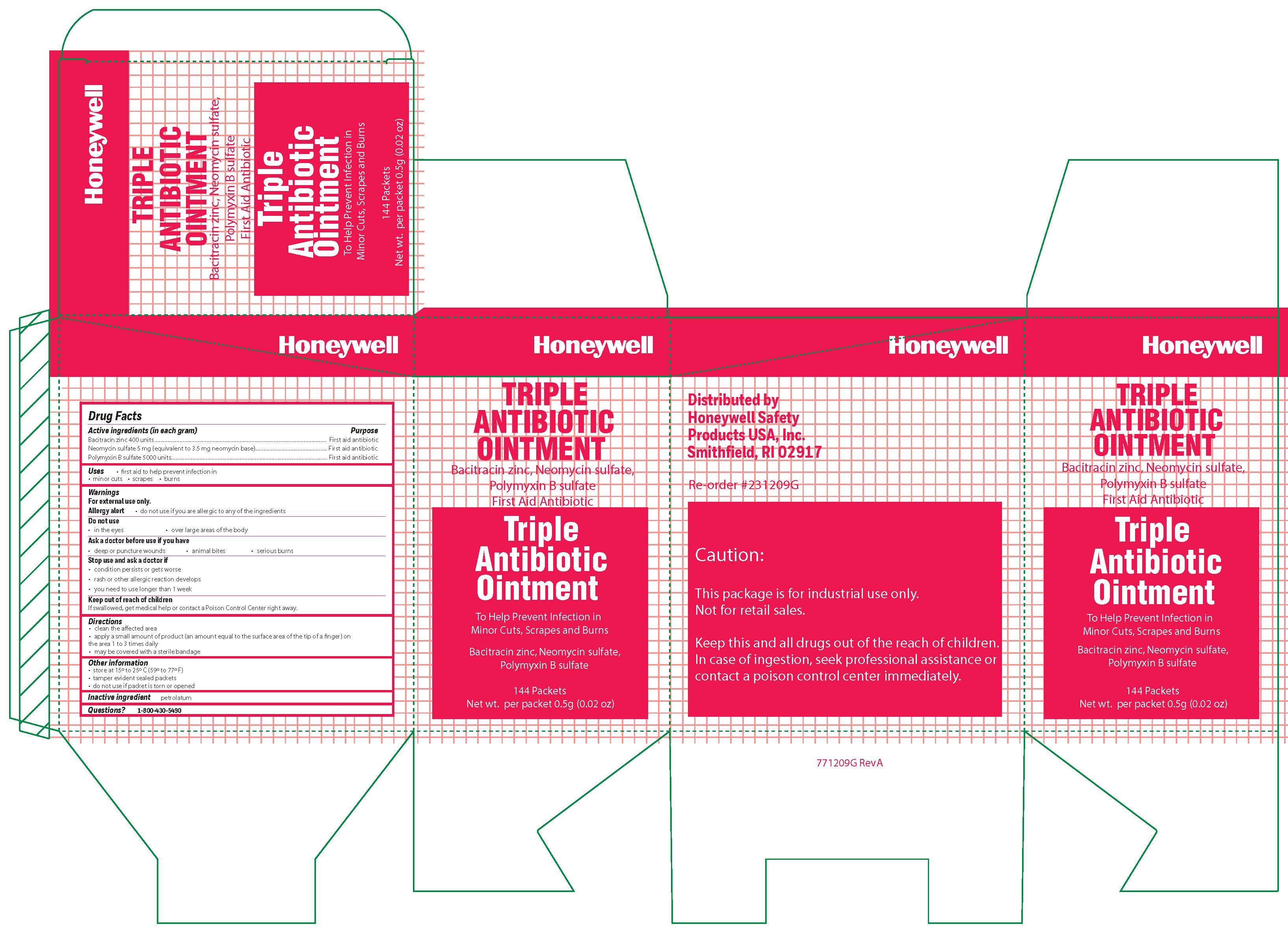
Triple
Active ingredient
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
BZK Wipe
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK Wipe
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
Hydrogen peroxide
Warnings
For external use only
Hydrogen peroxide
Directions
- clean the affected area
- spray a small amount of this product on the area 1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
4234
68180SIGMA Kit Contents
1 WOVEN 7/8 X 3 50/BOX
1 FNGERTP LRG WOVEN 25/BOX
1 FNGERTP WOVEN REG 40/BOX
1 WOVEN 2" X 3" 25/BOX
1 SWIFT KNUCKLE 40/BX
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 BURN JEL 1/8 OZ, 6 PER
1 NITRILE GLOVES 2PR BBP
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 HYDROGEN PEROXIDE SPRAY PUMP
1 GAUZE BANDAGE 4"X2 YDS STRETCH GZ
1 FIRST AID GUIDE ASHI
2 BLOODSTOPPER
1 NON ADHERENT PADS 2"X3" 50'S
1 COTTON BALLS STERILE 130
1 ANTISEPTIC WIPES BZK CHL 20'S
1 TRIPLE BIOTIC .5 GRAM PKT 20
2 COLD PACK 5"X9" BOXED
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 180 EMPTY BLANK NO LOGO
1 BANDAGE COMP 3" W/TELFA PAD 2
LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 TRI BNDG NON WOVEN 40"X40"X56"
| 4234 FIRST AID KIT
4234 first aid kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc. (118768815) |