GUARDANIA PAIN- capsaicin cream
LHP Pharma, Inc
----------
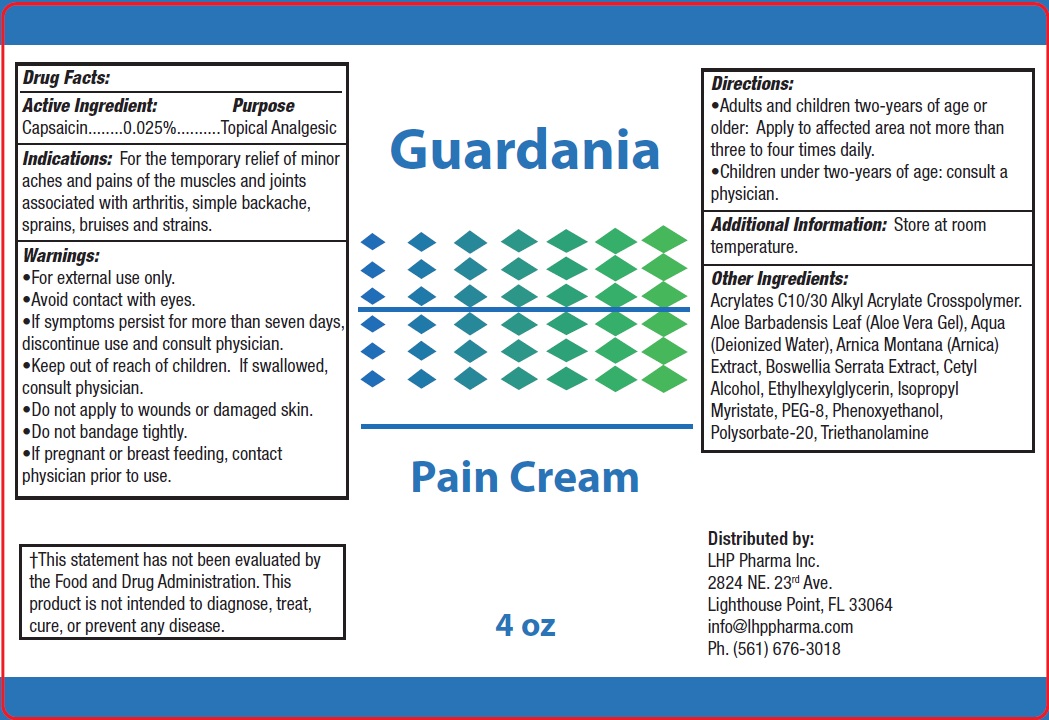
Guardania Pain Cream
Indications:
For the temporary relief of minor aches and pains of the muscles and joints associated with arthritis, simple backache, sprains, bruises and strains.
Warnings:
•For external use only.
•Avoid contact with eyes.
•If symptoms persist for more than seven days, discontinue use and consult physician.
Directions:
•Adults and children two-years of age or older: Apply to affected area not more than three to four times daily.
•Children under two-years of age: consult a physician.
| GUARDANIA PAIN
capsaicin cream |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - LHP Pharma, Inc (080702471) |
Revised: 10/2023
Document Id: 07241d6d-62d9-d588-e063-6294a90a925b
Set id: 84db196d-daf9-46f6-810a-f1aa48bf763a
Version: 2
Effective Time: 20231007
LHP Pharma, Inc