4229 FIRST AID KIT- 4229 first aid kit
Honeywell Safety Products USA, INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-4229: First Aid Kit ( alcohol wipes, ammonia, triple, aypanal, Pain stoppers- 68140FEDX)
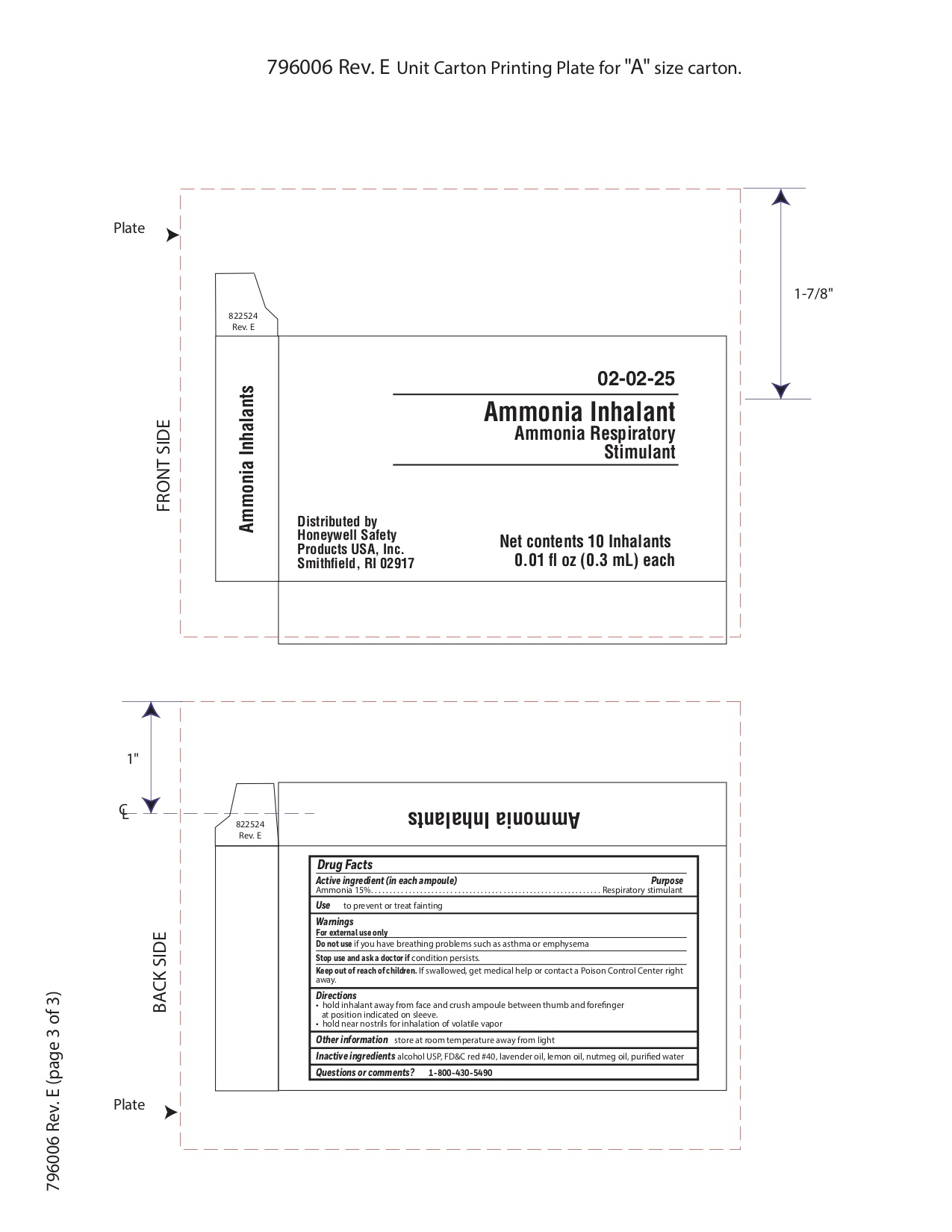
Ammonia Inhalent
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia Inhalent
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
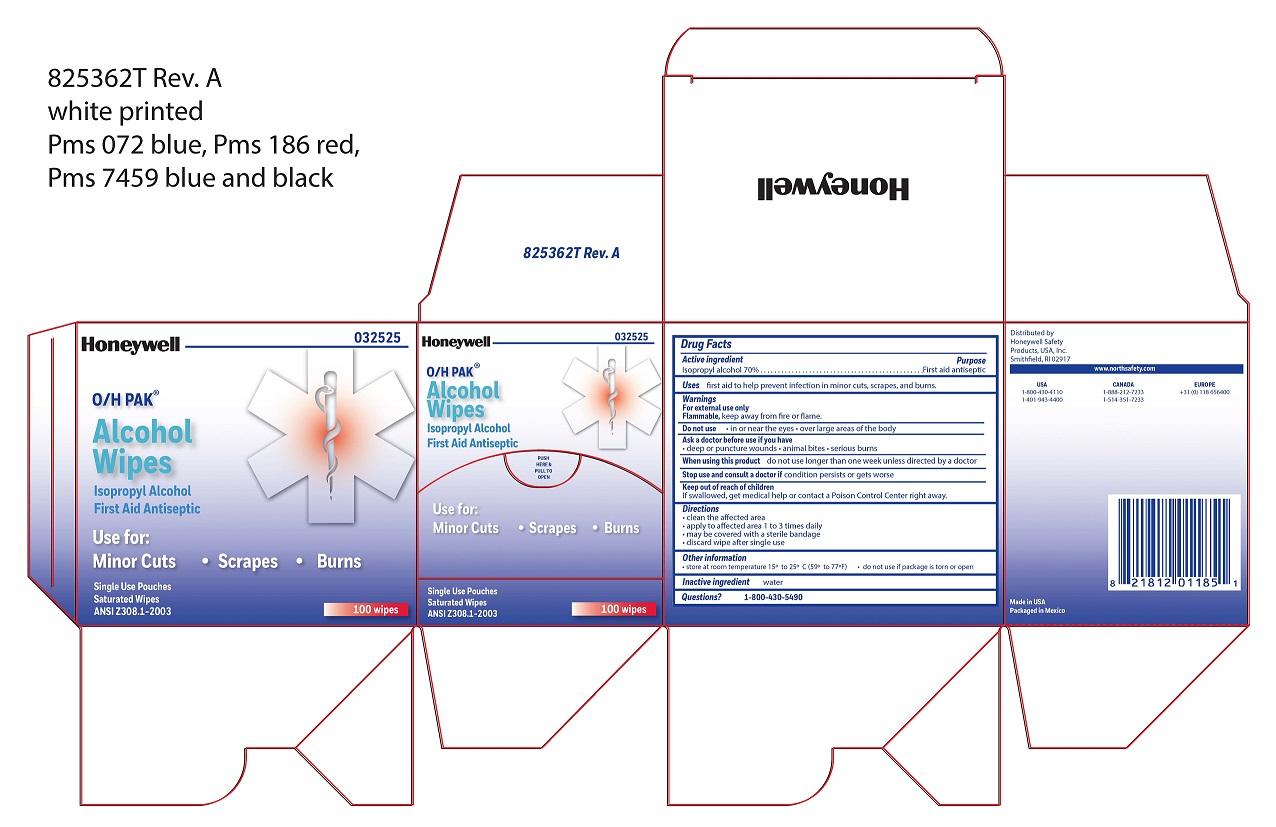
Alcohol Wipe
Directions
- clean the affected area
- apply wipe to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard wipe after single use
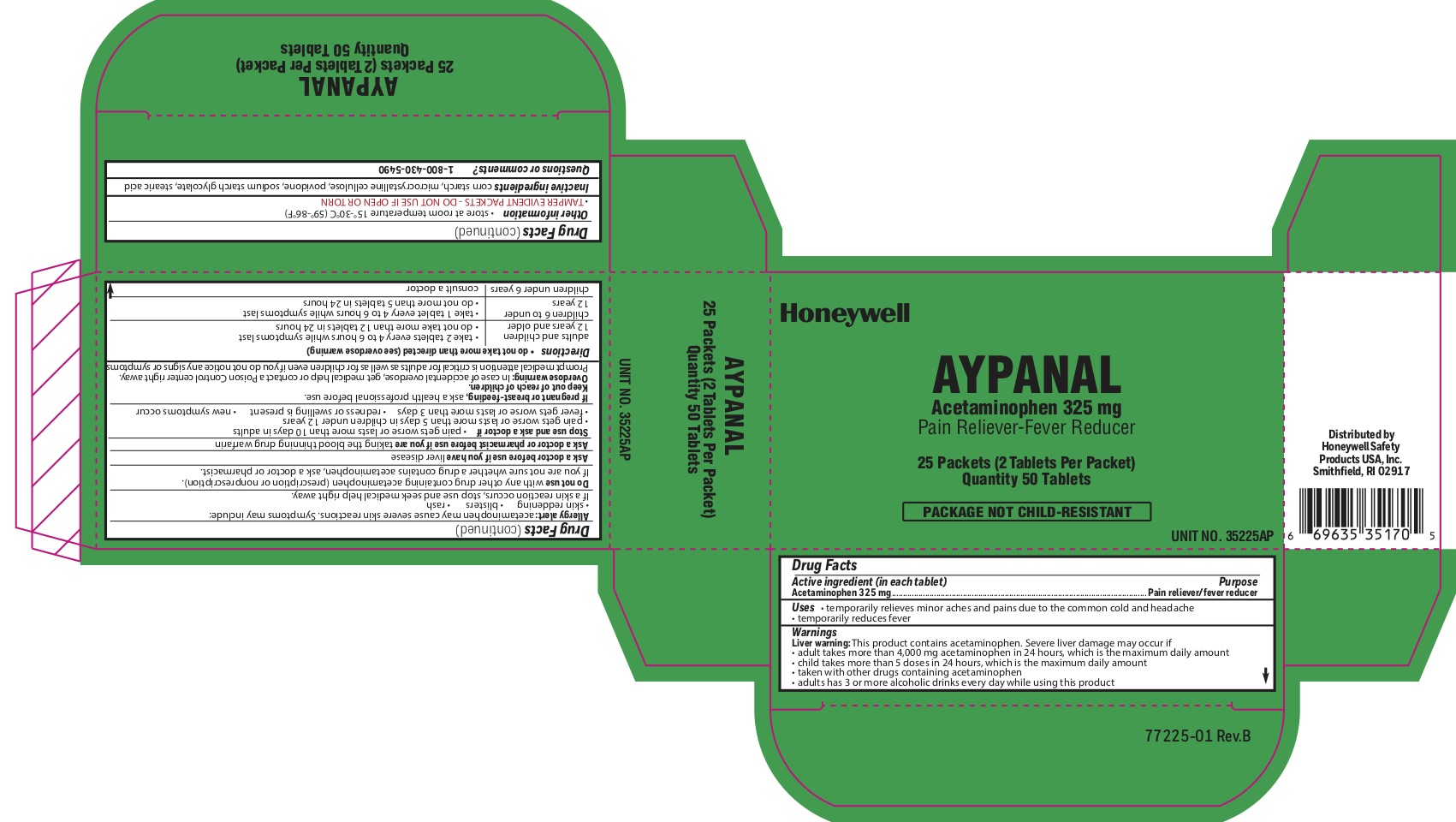
Aypanaly
Uses
- temporarily relieves minor aches and pains due to the common cold and headache - temporarily reduces fever
Aypanal
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product:
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
- If a skin rash occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Aypanal
Directions
do not take more than directed (see overdose warning)
adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than 12 tablets in 24 hours
children 6 to under 12 years of age
- take 1 tablet every 4-6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
children under 6 years consult a doctor
Aypanal
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
Aypanal
Inactive ingredients
corn starch, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
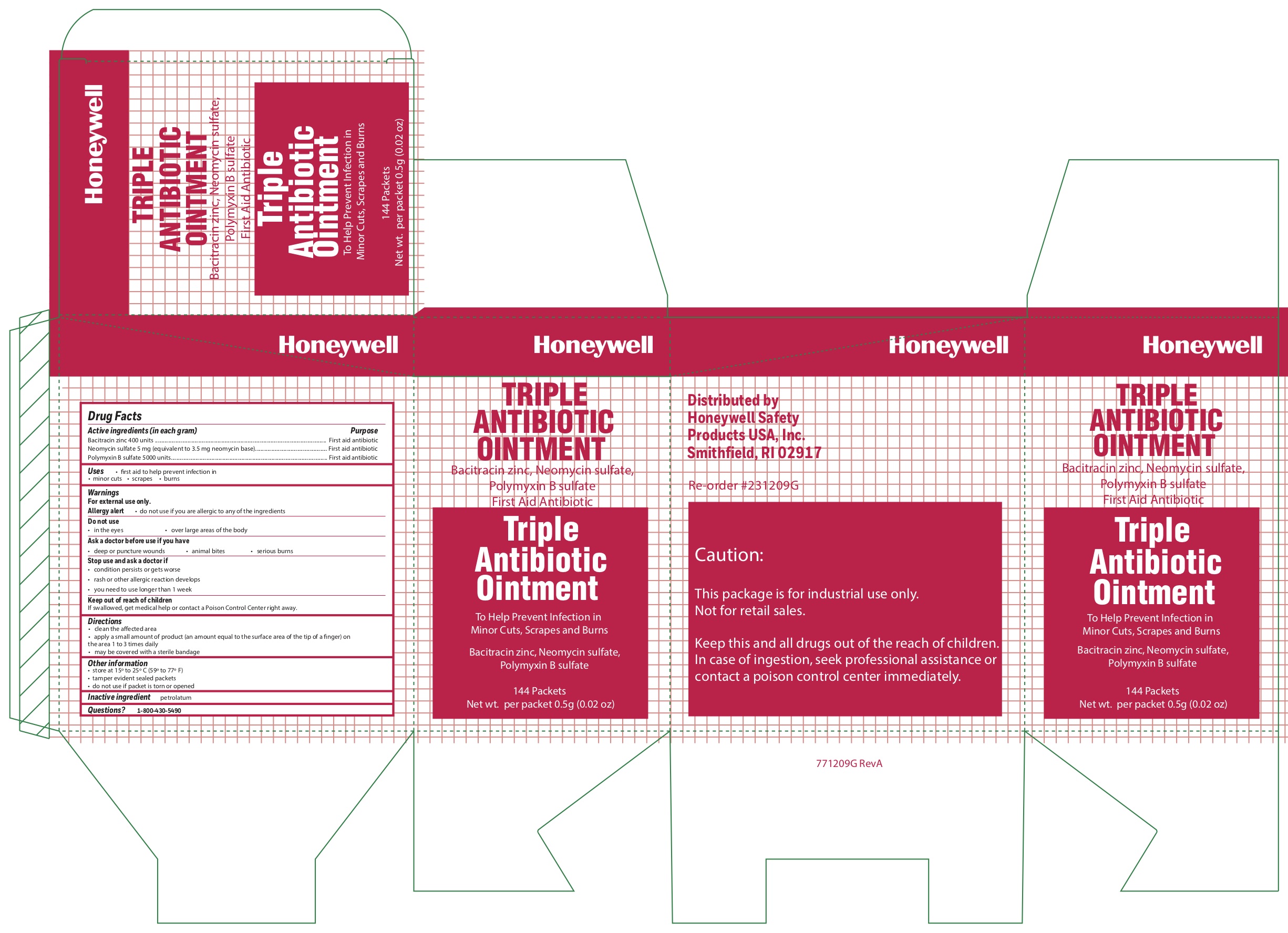
Triple
Active ingredient
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert do not use if you are allergic to any of the ingredients
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 o to 25 oC (59 o to 77 o F)
- tamper evident sealed packets
- do not use if packet is torn or opened
Pain Stoppers
Active ingredient
Acetaminophen 110mg
Aspirin 162mg (NSAID)*
Caffeine 32.4mg
Salicylamide 152mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Pain Stoppers
Purpose
Pain reliever/fever reducer
Pain reliever/fever reducer
Diuretic
Pain reliever/fever reducer
Pain Stoppers
Uses
for the temporary relief of minor aches and pains due to:
• common cold
• headache
• muscular aches
• premenstrual and menstrual cramps
Pain Stoppers
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
more than 12 tablets in 24 hours, which is the maximum daily amount
with other drugs containing acetaminophen
3 or more alcoholic drinks every day while using this product
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you are taking a diuretic
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
Stop use and ask a doctor if
- symptoms do not improve
- new symptoms occur
- pain or fever persists or gets worse
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pin that does not get better
- if ringing in the ears or a loss of hearing occurs, consult a doctor before taking any more of this product.
Pain Stoppers
Directions
- adults and children 12 years of age and over, take 2 tablets every 4 hours while symptoms persist
- do not take more than 12 tablets in 24 hours
- children under 12 years: consult a doctor
Pain Stoppers
Other information
store at a controlled room temperature 15
0 -30
0 C (59
0 -86
0 F)
TAMPER EVIDENT-DO NOT USE IF OPEN OR TORN
Pain Stoppers
Inactive ingredients
FD&C Yellow #6, magnesium stearate, microcrystalline cellulose, povidone, starch, stearic acid,
4229
68140FEDX Kit Contents
1 WOVEN 2" X 3" 25/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 1X3 WOVEN SING 50/BOX
1 SWIFT KNUCKLE 40/BX
1 AMMONIA INHALANTS 10 PER
1 NITRILE GLOVES 2PR BBP
1 ADHESIVE TAPE W/P 1" X 10YDS
1 GAUZE BANDAGE 2"X2 YDS STRETCH GZ
1 FIRST AID GUIDE ASHI
1 BLOODSTOPPER
1 CTA STRL 6" SGL TIP 12/2
1 ALCOHOL WIPES 50'S
1 AYPANAL NON-ASP IND 2/ENV 100
1 PAIN STOPPERS IND PK 2ENV 250
1 TRIPLE BIOTIC .5 GRAM PKT 20
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 F A KIT EMPTY BLANK 140
LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
1 TRI BNDG NON WOVEN 40"X40"X56"
| 4229 FIRST AID KIT
4229 first aid kit kit |
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, INC (118768815) |