4224 FIRST AID KIT- 4224 first aid
Honeywell Safety Products USA, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-4224: First Aid Kit (Ammonia Inh, EW, Burn Jel, PVP Wipes, ASA- 35824CG)
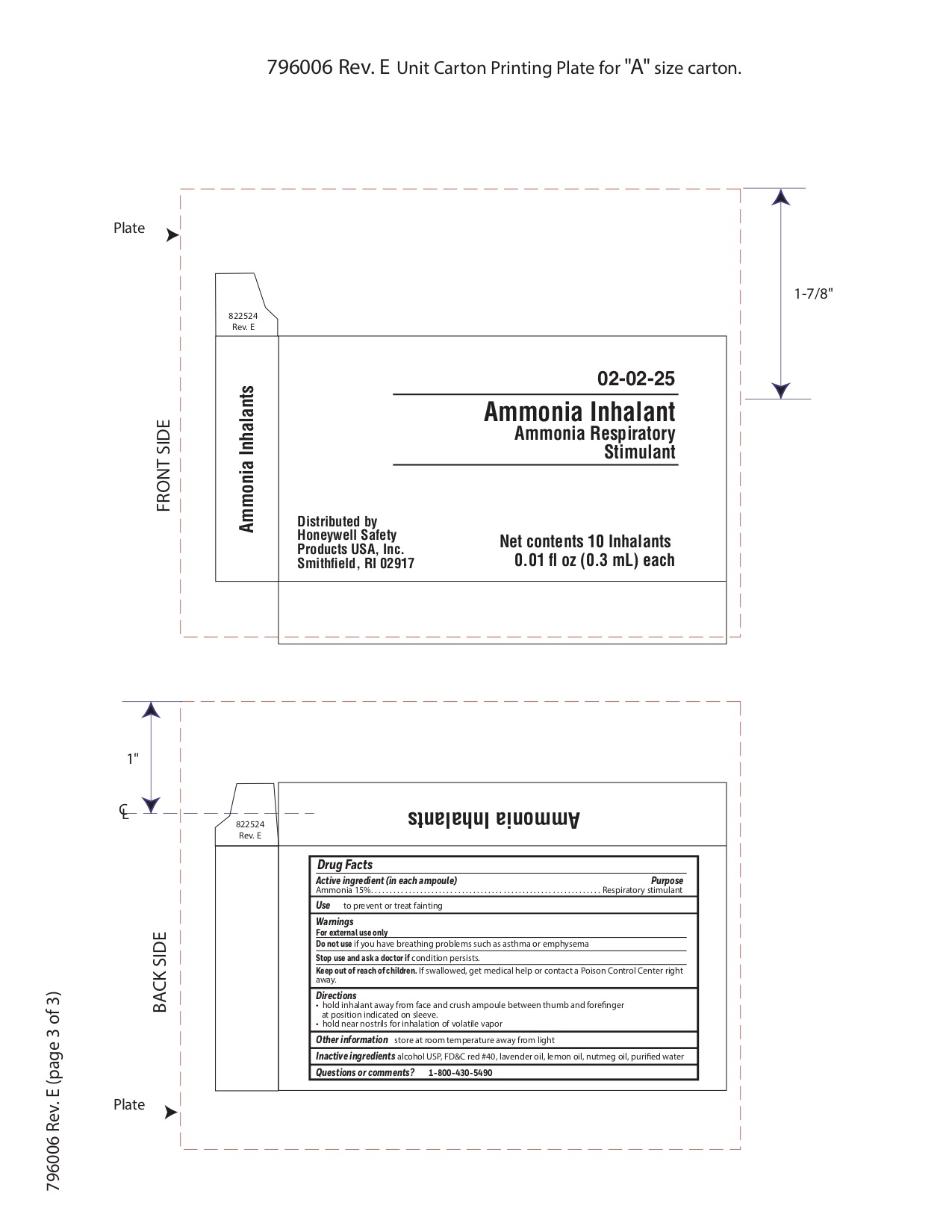
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
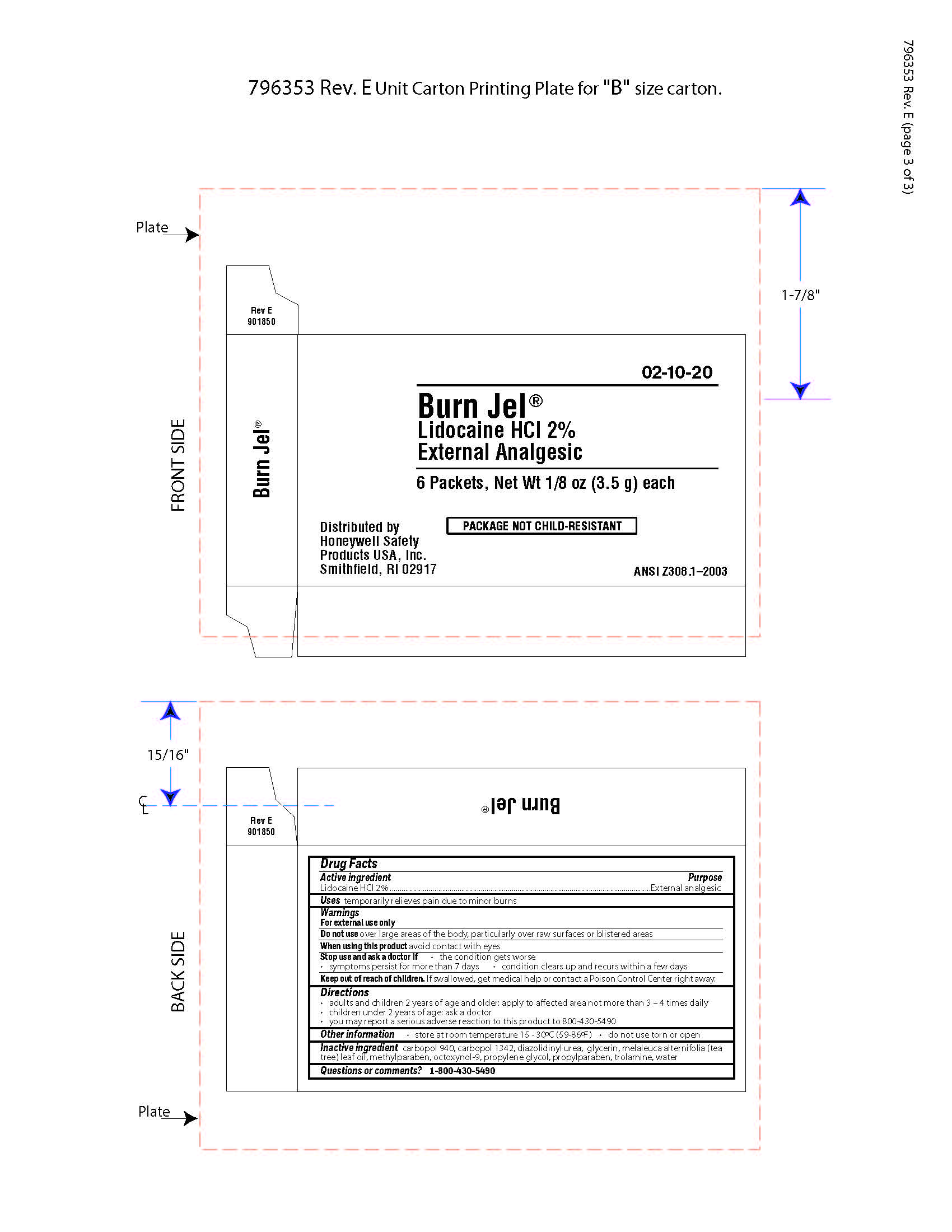
Burn Jel
Warnings
For external use only
Burn JEl
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
PVP
Warnings
For external use only
PVP
Directioons
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
PVP
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
Aspirin
Uses
temporarily reduces fever and relieves minor aches and pains associated with:
- a cold
- headache
- toothache
- muscular aches
- backache
- minor pain of arthritis
- premenstrual and menstrual periods
Aspirin
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning:
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are
taking a prescription drug for diabetes, gout or arthritis
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- ringing in the ears or loss of hearing occurs
- any new symptoms appear
If pregnant or breast-feeding
If pregnant or breat-feeding, ask a health professional before use. It is especially important not to use aspirin during the last three months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Aspirin
Directions
- drink a full glass of water with each dose
- adults and children 12 years of age and older: take 1 or 2 tablets every 4 hours while symptoms last, not more than 12 tablets in 24 hours
- children under 12 years of age: consult a doctor
Aspirin
Other information
store at room temperature 15° - 30°C (59° - 86°F)
TAMPER EVIDIENT PACKETS - DO NOT USE IF OPEN OR TORN
Aspirin
Inactive ingredients
corn starch, croscarmellose sodium*, hypromellose*, microcrystalline cellulose*, mineral oil*, polyethylene glycol*, povidone, propylene glycol, silicon dioxide, stearic acid*, titanium dioxide*
*may contain these ingredients
4224
35824CG Kit Contents
1 AMMONIA INHALANTS 10 PER
2 GAUZE BANDAGE, 4" X 6 YD
1 TOURNIQUET, 1 PER
1 WIRE SPLINT 1 PER
2 GAUZE COMPRESS, 1728 SQ IN 1
2 BUFFERED EYE WASH 1 OZ BTL
2 ADHESIVE BDG,PLSTIC,1"X3"16PER
2 BURN JEL 1/8 OZ, 6 PER
1 PVP IODINE WIPES 10 PER
2 BANDAGE COMP 2" W/TELFA PAD 4
3 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
4 BAYER 12 PACK PER ZIP BAG
24 UNIT PLASTIC HIPS MT BLANK
1 ADHES TAPE EYE STRIPS 2'S
2 TRI BNDG NON WOVEN 40"X40"X56"
| 4224 FIRST AID KIT
4224 first aid kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc. (118768815) |