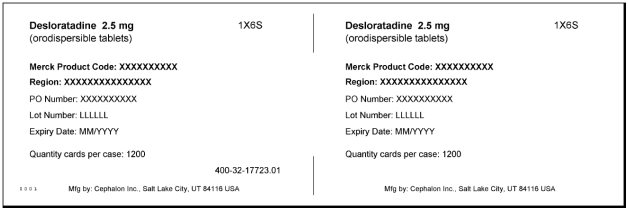
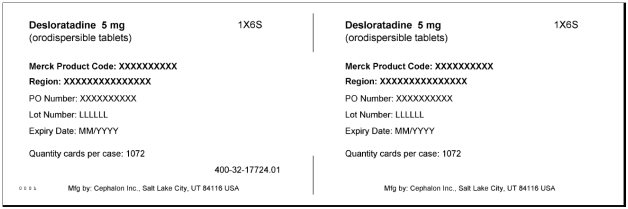
DESLORATADINE- desloratadine tablet, orally disintegrating
Anesta Corporation
----------
Desloratadine Orally disintegrating tablet
| DESLORATADINE
desloratadine tablet, orally disintegrating |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| DESLORATADINE
desloratadine tablet, orally disintegrating |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Anesta Corporation (177319472) |
Revised: 5/2016
Document Id: 3702bd7b-430c-46ca-8222-9e70dcb5417d
Set id: 84b20dcb-e15b-44a2-b692-1eea5adb996e
Version: 3
Effective Time: 20160527
Anesta Corporation