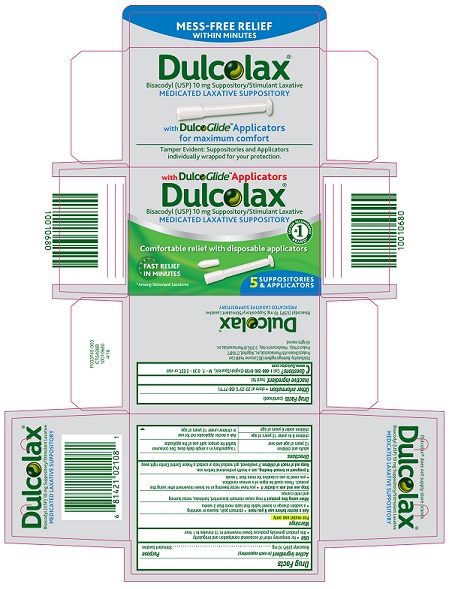
DULCOLAX- bisacodyl suppository
Boehringer Ingelheim Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dulcolax®
Bisacodyl
(USP) 10 mg Suppository/Stimulant Laxative
Medicated Laxative
Suppository
with DulcoGlide™Applicators
- for temporary relief of occasional constipation and irregularity
- this product generally produces bowel movement in 15 minutes to 1 hour
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
| adults and children 12 years of age and over | 1 suppository in a single daily dose. See consumer leaflet for proper, safe use of the applicator. |
| children 6 to under 12 years of age | Ask a doctor. Applicator not for use in children under 12 years of age. |
| children under 6 years of age |
Questions? call 1-888-285-9159 (English/Spanish), M – F, 8:30 – 5 EST, or visit www.Dulcolax.com
Distributed by: Boehringer Ingelheim (BI) Consumer Health Care Products Division of BI Pharmaceuticals, Inc., Ridgefield, CT 06877. Product of Italy. Manufactured in Italy. © 2015, Boehringer Ingelheim Pharmaceuticals, Inc. All rights reserved.
| DULCOLAX
bisacodyl suppository |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
Revised: 10/2021
Document Id: b0528a9d-1356-4d07-8331-4750c0ef1f23
Set id: 8459ee71-64eb-daba-d73d-b6ee37b945d1
Version: 4
Effective Time: 20211029
Boehringer Ingelheim Pharmaceuticals, Inc.