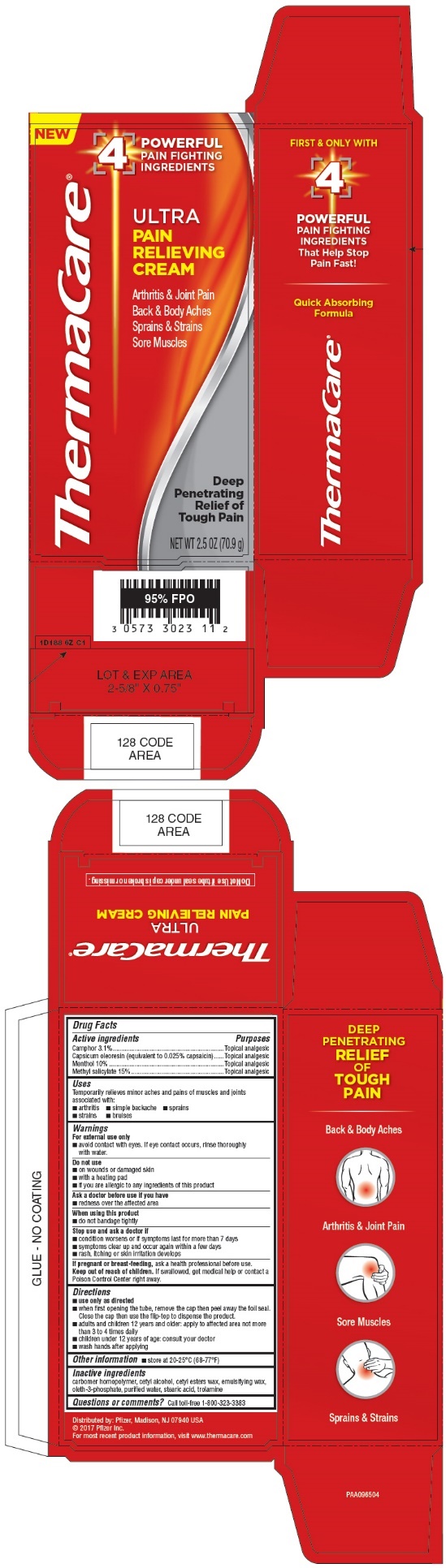
THERMACARE ULTRA- camphor, capsicum oleresin, menthol, methyl salicylate cream
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Active Ingredients
Camphor 3.1%
Capsicum oleoresin (equivalent to 0.025% capsaicin)
Menthol 10%
Methyl salicylate 15%
Uses
Temporarily relieves minor aches and pains of muscles and joints associated with:
- •
- arthritis
- •
- simple backache
- •
- sprains
- •
- strains
- •
- bruises
Warnings
For external use only
- •
- avoid contact with eyes. If eye contact occurs, rinse thoroughly with water.
Do not use
- •
- on wounds or damaged skin
- •
- with a heating pad
- •
- if you are allergic to any ingredients of this product
Directions
- •
- use only as directed
- •
- when first opening the tube, remove the cap then peel away the foil seal. Close the cap then use the flip-top to dispense the product.
- •
- adults and children 12 years and older: apply to affected area not more than 3 to 4 times daily
- •
- children under 12 years of age: consult your doctor
- •
- wash hands after applying
| THERMACARE ULTRA
camphor, capsicum oleresin, menthol, methyl salicylate cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |
Revised: 3/2021
Document Id: 9695df96-6bc5-4c5a-a11e-e2cc2d7e9ec8
Set id: 844f8462-c8b7-4ace-b9b3-e7b05795054a
Version: 3
Effective Time: 20210322
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC