4208 FIRST AID KIT- 4208 first aid kit
Honeywell Safety Products USA, INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-4208: First Aid Kit (FABC, Amm Inh, EW, PVP wipes- 019736-0023L)
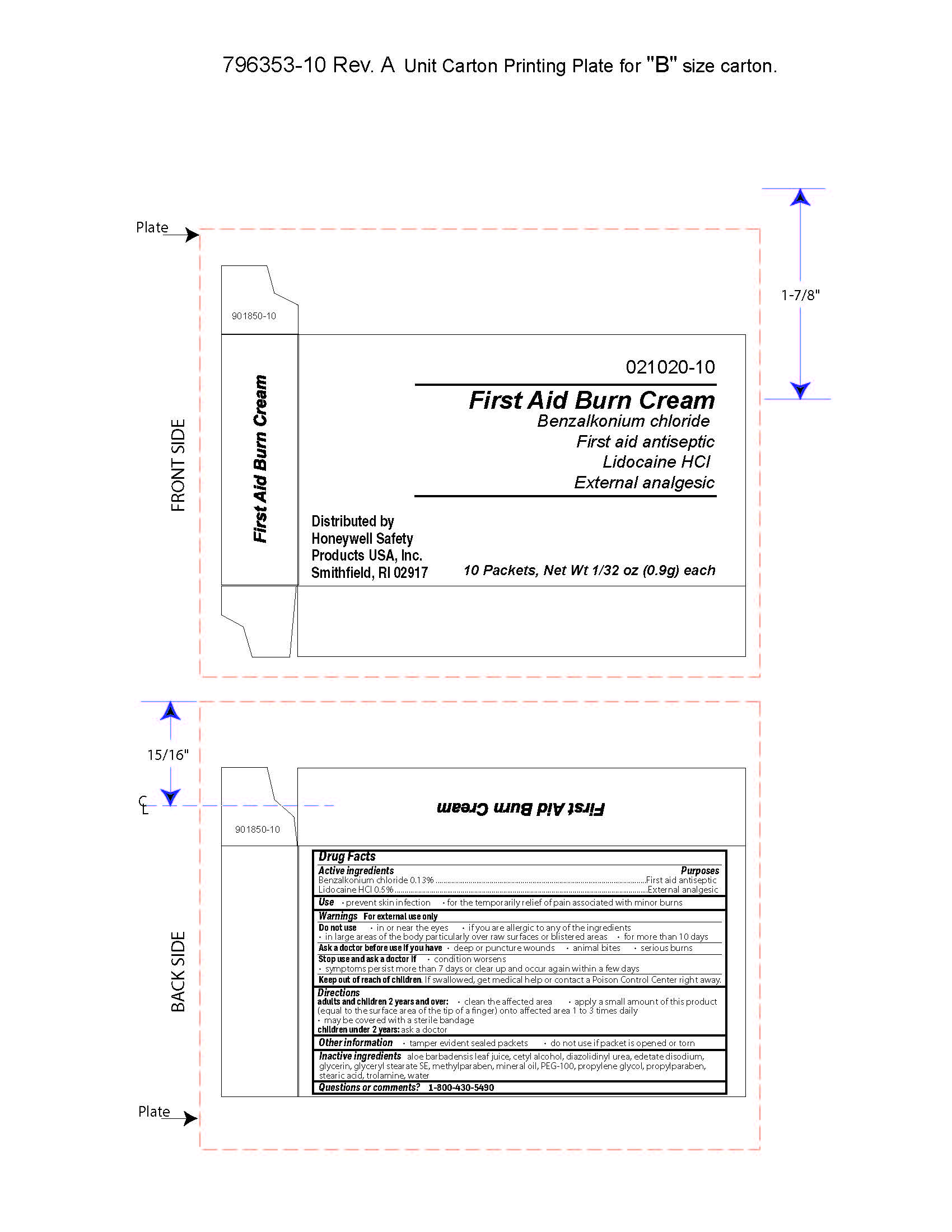
First Aid Burn Cream
Uses
- prevent skin infection
- for temporary relief of pain associated with minor burns
First Aid Burn Cream
Warnings
For external use only
First Aid Burn Cream
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
First Aid Burn Cream
Other information
- tamper evident sealed packets
- do not use if packet is opened or torn
First Aid Burn Cream
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
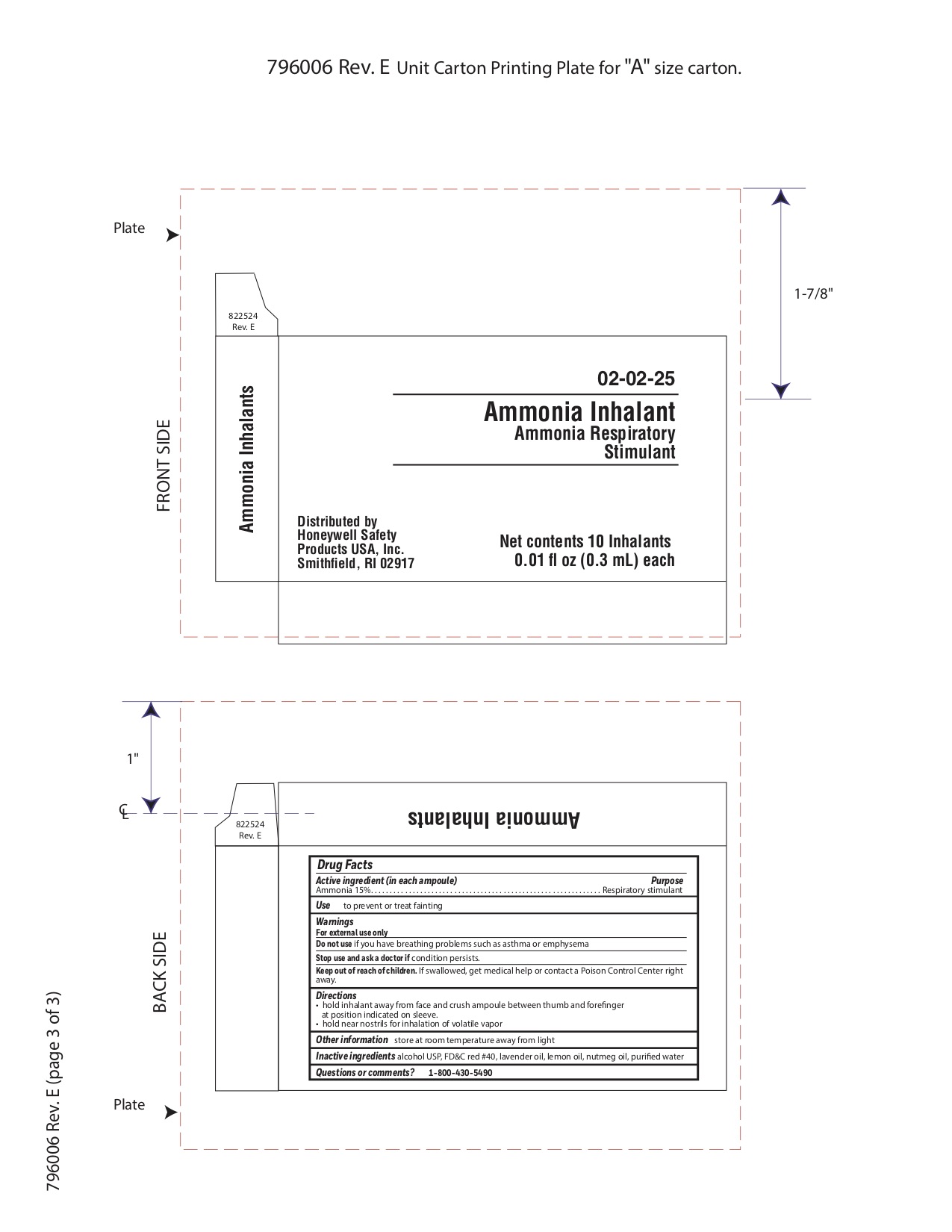
Ammonia Inhalent
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia Inhalent
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
PVP
Warnings
For external use only
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away
PVP
Directions
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
PVP
Other informatiion
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
4208
019736-0023L Kit Contents
1 KNUCKLE BAND 8 PER
3 FIRST AID BURN CREAM 6 PER
1 AMMONIA INHALANTS 10 PER
2 EYE DRESS PKT W/4 ADH STRIPS
1 GAUZE PADS, 3" X 3", 4 PER
1 ADHESIVE TPE 1"X2-1/2 YD 2 PER
1 GAUZE BANDAGE, 2" X 6 YD,2 PER
2 BUFFERED EYE WASH 1 OZ BTL
1 BANDAGE COMP, 2" OFFSET, 4 PER
1 BANDAGE COMP, 4" OFFSET, 1 PER
4 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 ADH BAND, EXTRA LARGE, 6 PER
2 PVP IODINE WIPES 10 PER
1 NITRILE GLOVES 2PR BBP
1 TWEEZER PLASTICS 4"
1 FIRST AID GUIDE ASHI
LBL STOCK 6-3/8"X4"
LBL STOCK 3"x1-7/8"
1 LABEL NORTH CONTENTS 8X8 ID B
1 KIT STL 24 UN WHITE 01
1 LABEL 24U CVR NORTH WELDERS
| 4208 FIRST AID KIT
4208 first aid kit kit |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, INC (118768815) |