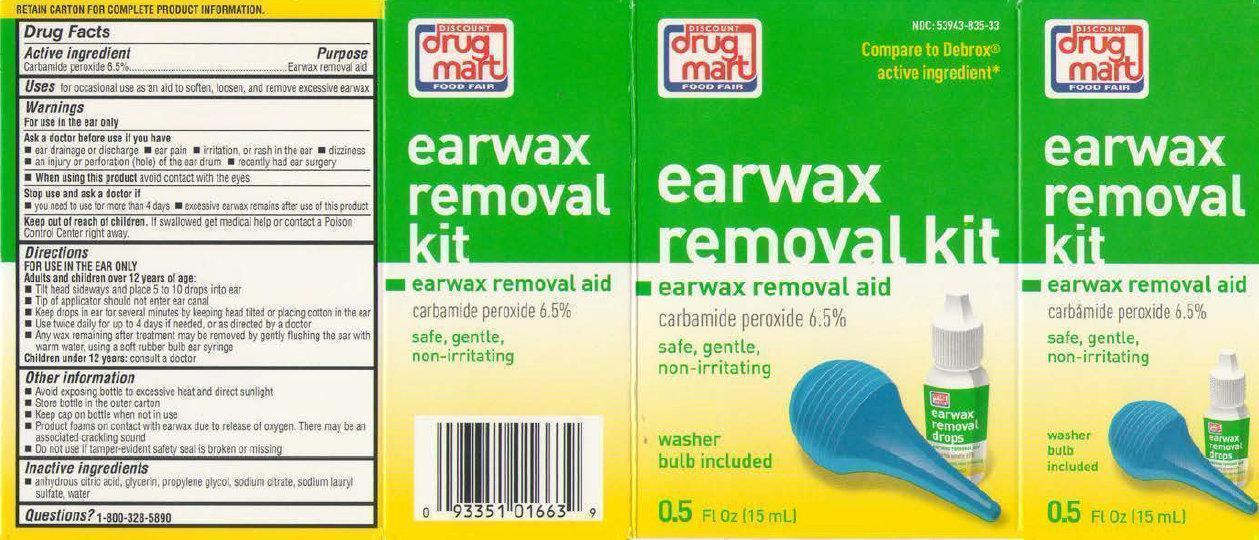
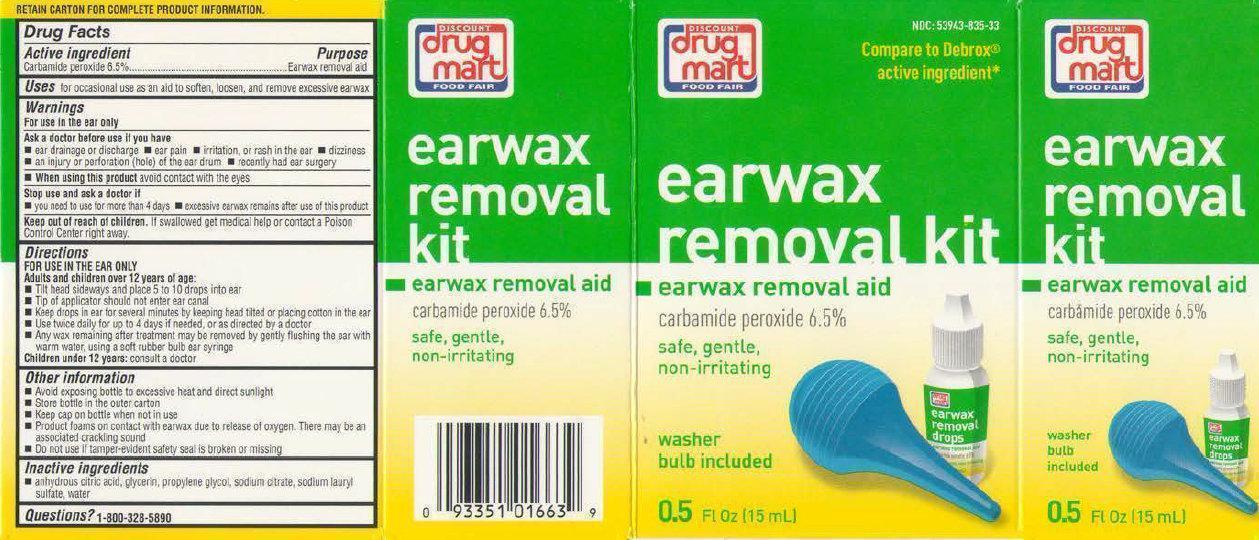
Label: DISCOUNT DRUG MART FOOD FAIR EARWAX REMOVAL KIT- carbamide peroxide solution
- NDC Code(s): 53943-835-33
- Packager: Discount Drug Mart
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug facts
- Active ingredient
- Uses
-
Warnings
For use in the ear only
Ask a doctor before use if you have
- Ear drainage or discharge
- Ear pain
- Irritation, or rash in the ear
- Dizziness
- An injury or perforation (hole) of the ear drum
- Recently had ear surgery
-
Directions
FOR USE IN THE EAR ONLYAdults and children over 12 years of age:
Children under 12 years: consult a doctor
- Tilt head sideways and place 5 to 10 drops into ear
- Tip of applicator should not enter ear canal
- Keep drops in ear for several minutes by keeping head titled or placing cotton in the ear
- Use twice daily for up to 4 days if needed, or as directed by a doctor
- Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe
- Other Information
- Inactive ingredients
- Questions?
-
SPL UNCLASSIFIED SECTION
*This product is not manufactured or distributed by Prestige Brands Inc. owner of the registered trademark Debrox.
If Dissatisfied, Return Unused Portion And Package To Store Where Purchased. If Unable To Return To Store, Send Reason For Dissatisfaction. Name, Address And Empty Package To: Discount Drug Mart, 211 Commerce Drive, Median Ohio 44256 Satisfaction Guaranteed
Distributed By: Drug Mart-Food Fair 211 Commerce Dr., Median, Ohio 44256 Made in the USA
- PRINCIPAL DISPLAY PANEL
- Product Labels
-
INGREDIENTS AND APPEARANCE
DISCOUNT DRUG MART FOOD FAIR EARWAX REMOVAL KIT
carbamide peroxide solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53943-835 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM LAURYL SULFATE (UNII: 368GB5141J) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53943-835-33 1 in 1 CARTON 11/27/2014 1 15 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 11/27/2014 Labeler - Discount Drug Mart (047741335) Establishment Name Address ID/FEI Business Operations Bell Pharmaceuticals, Inc. 140653770 manufacture(53943-835)