4188 FIRST AID KIT- 4188 first aid
Honeywell Safety Products USA, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-4188: First Aid Kit (Burn Jel, Ammonia Inh) 10704-4299
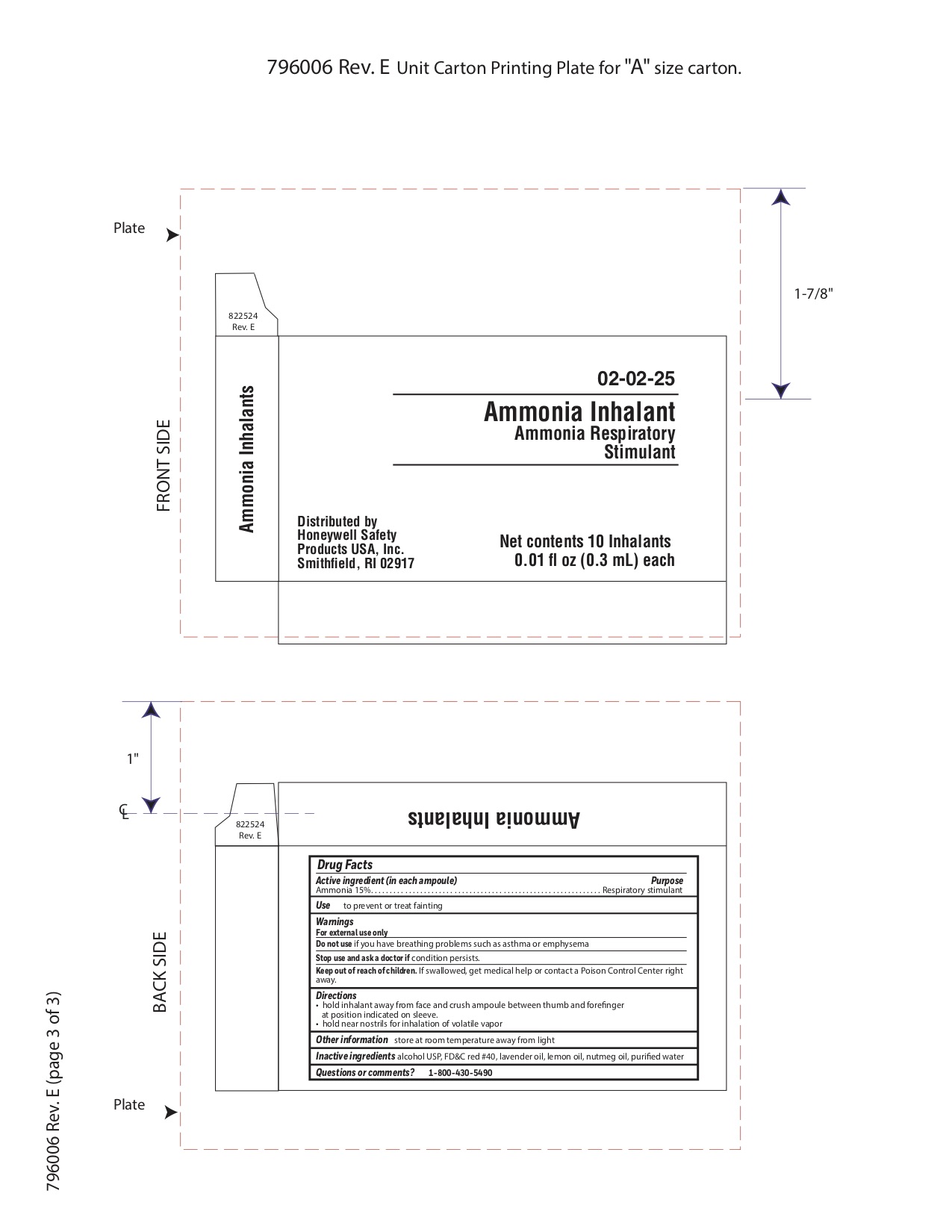
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
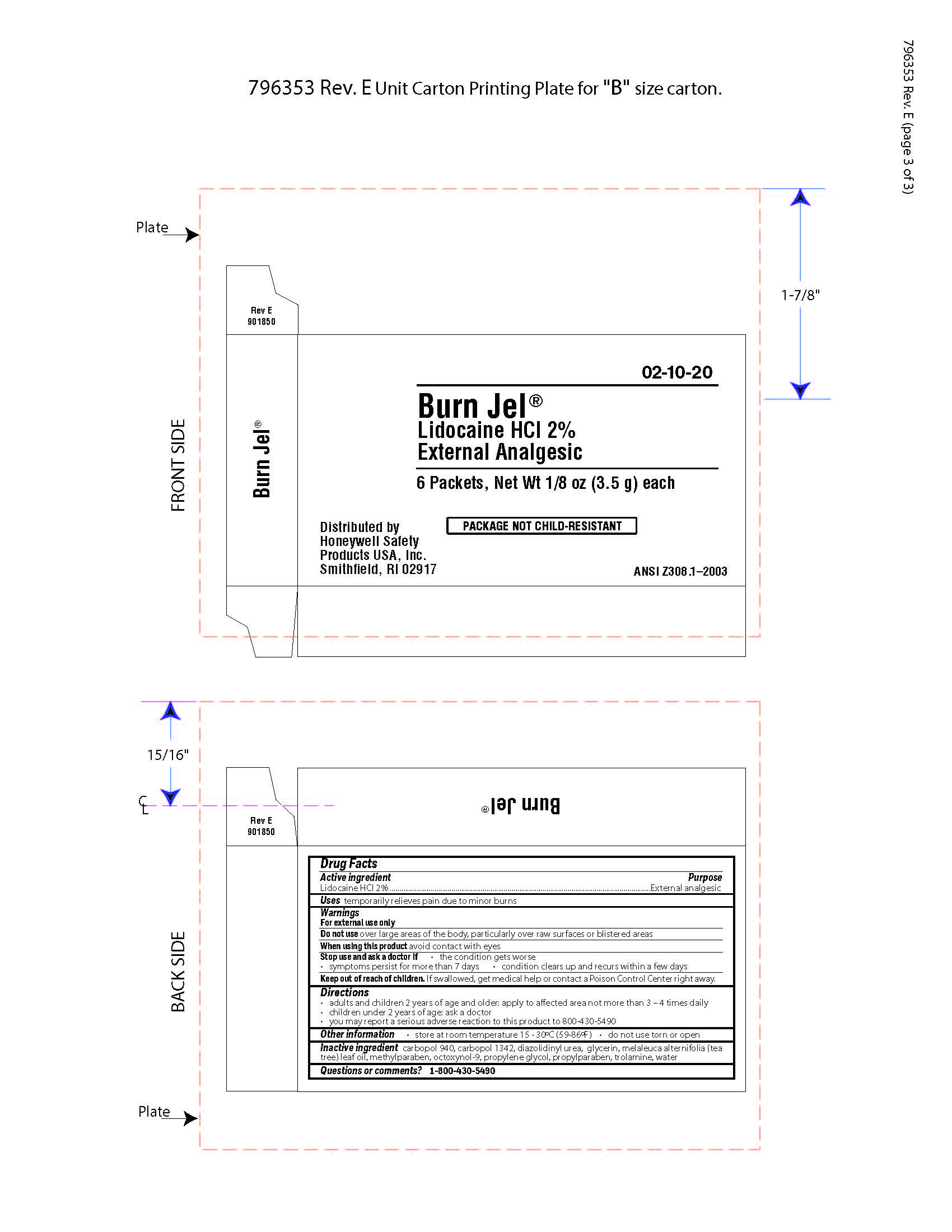
Burn Jel
Warnings
For external use only
Burn JEl
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
4188
010704-4299 Kit Contents
2 AMMONIA INHALANTS 10 PER
2 TOURNIQUET, 1 PER
15 TRIANGULAR BDG, NON-STERILE
4 BANDAGE COMP, 2" OFFSET, 4 PER
8 BANDAGE COMP, 4" OFFSET, 1 PER
1 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 BURN JEL 1/8 OZ, 6 PER
LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 36 UN WHT 01 HOR SHELF
1 LABL INSTR 24 & 36 UNIT KITS
| 4188 FIRST AID KIT
4188 first aid kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc. (118768815) |