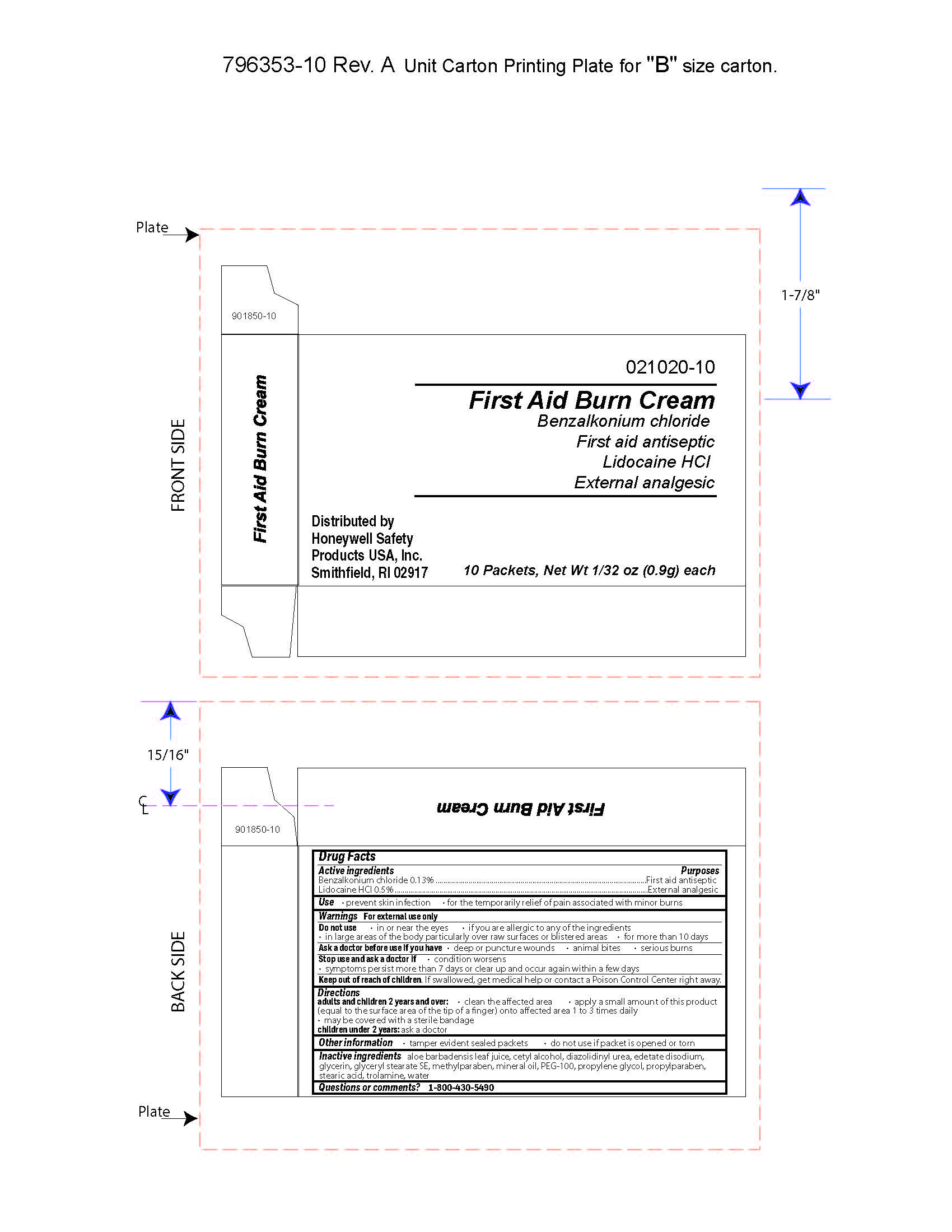
First Aid Burn Cream
Active ingredient
Benzalkonium chloride o.13%
Lidocaine HCl 0.5%
First Aid Burn Cream
Purpose
First aid antiseptic
External analgesic
First Aid Burn Cream
Uses
- prevent skin infection
- for temporary relief of pain associated with minor burns
First Aid Burn Cream
Warnings
For external use only
Do not use
- in or near the eyes
- if you are allergic to any of the ingredients
- in large areas of the body, particularly over raw surfaces or blistered areas
- for more than 10 days
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occurs again within a few days
First Aid Burn Cream
Directions
-
adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
First Aid Burn Cream
Other information
- tamper evident sealed packets
- do not use if packet is opened or torn
First Aid Burn Cream
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
First Aid Burn Cream
Questions
1-800-430-5490
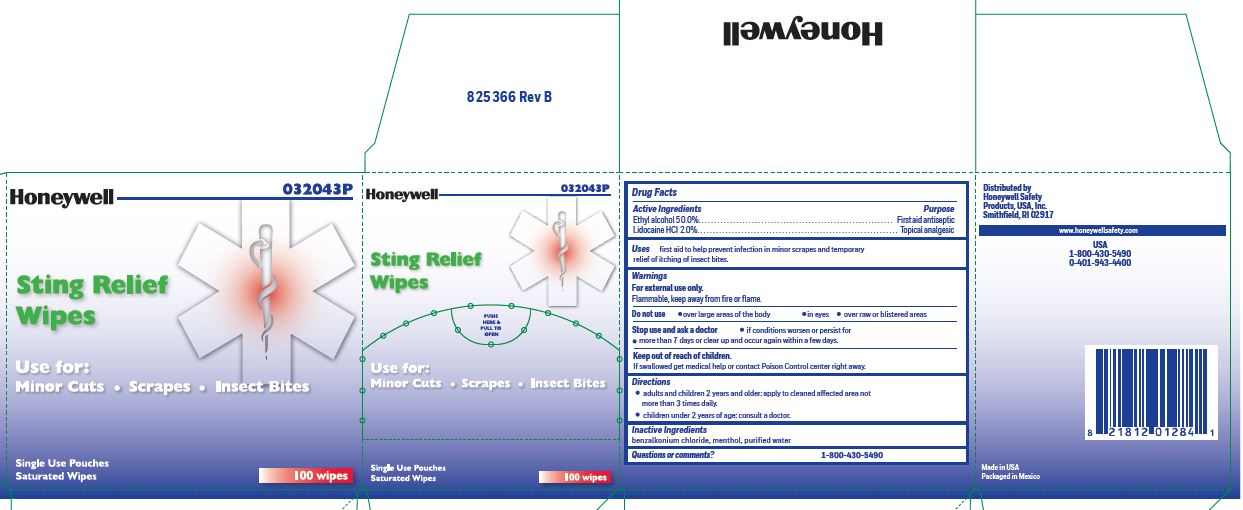
Sting Relief
Active ingredient
Ethyl alcohol 50.0%
Lidocaine HCl 2.0%
Sting Relief
Purpose
Antiseptic
Topical pain relief
Sting Relief
Uses
- prevent infection in minor scrapes, and temporary relief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable, keep away from open fire or flame
Do not use
- over large areas of the body
- in eyes
- over raw or blistered areas
Stop use and ask a doctor
- if conditions worsen or persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Sting Relief
Directions
-
adults and children 2 years and older: Apply to cleaned affected area not more than 3 times daily.
- children under 2 years of age: consult a doctor.
Sting Relief
Inactive ingredients
benzalkonium chloride, menthol, and purified water
Sting relief
Questions or Comments
1-800-430-5490
4187
010552-3305 Kit Contents
1 KNUCKLE BAND 8 PER
1 FIRST AID BURN CREAM 6 PER
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 GAUZE COMPRESS, 1728 SQ IN 1
1 INSTANT COLD PACK 4" X 6"
1 BUFFERED EYE WASH 1 OZ BTL
1 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 BURN JEL 1/8 OZ, 6 PER
2 ALCOHOL PREP PADS 10P
1 PVP IODINE WIPES 10 PER
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 16 UN (VERTICAL)
1 LABL INSTR FA REV A
1 STING Relief WIPES 10
1 AYPANAL NON-ASP 25/2
Eyesaline
Active ingredient
Sterile Water 99%
Eyesaline
Purpose
Eyewash
Eyesaline
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyesaline
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Stop use and ask a doctor if
- you experience eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Eyesaline
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyesaline
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
Eyesaline
Questions
1-800-430-5490 Honeywell Sadety Products USA, Inc. Smithfield, RI 02917
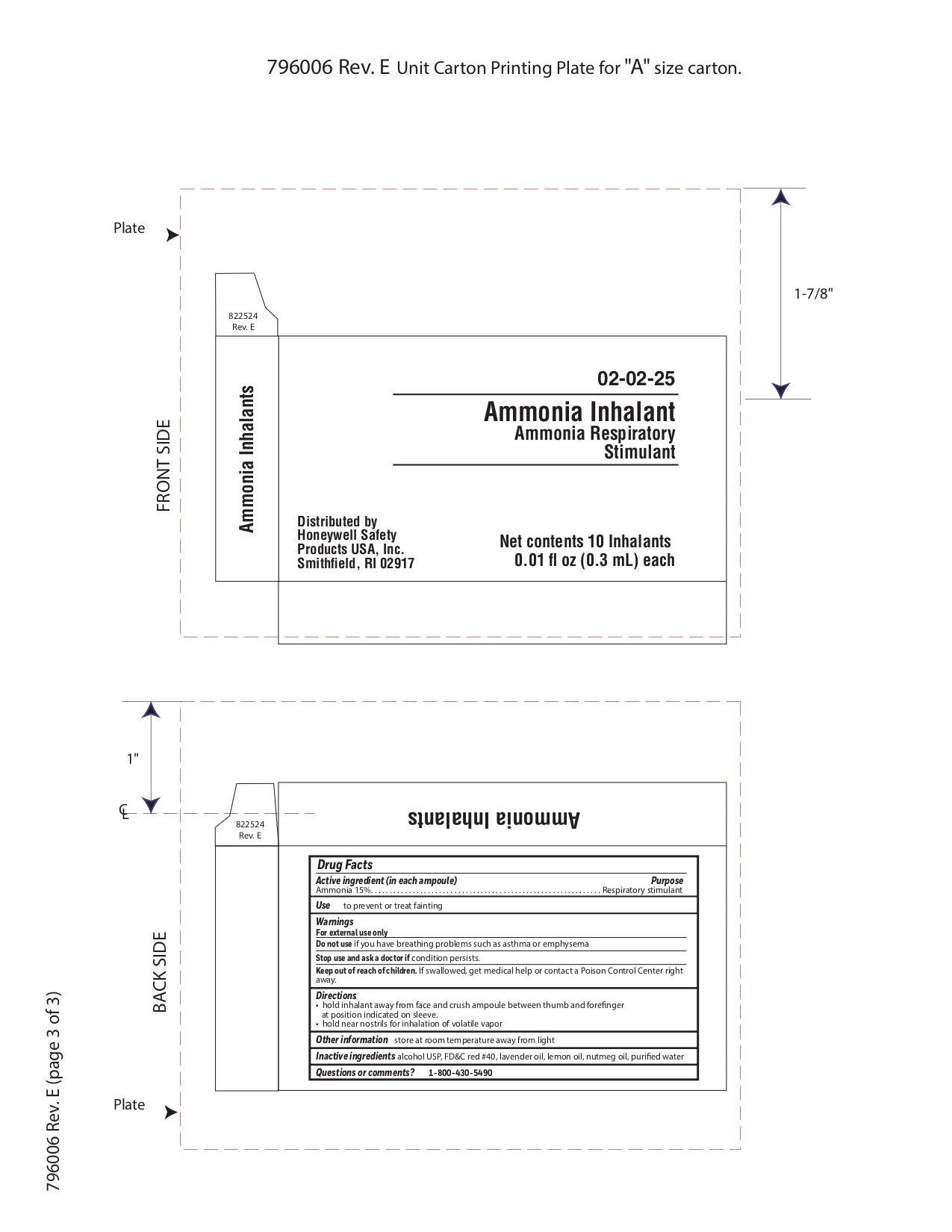
Ammonia Inhalent
Active ingredient
Ammonia 15%
Ammonia Inhalent
Purpose
Respiratory stimulant
Ammonia Inhalent
Uses
- to prevent or tret fainting
Ammonia Inhalent
Warnings
For external use only
Do not use
- if you have breathing problems such as asthma or emphysema
Stop use and ask a doctor if
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Ammonia Inhalent
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia Inhalent
Other information
- store at room temperature away from light
Ammonia Inhalent
Other information
- store at room temperature away from light
Ammonia Inhalent
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Ammonia Inhalent
Questions or Comments
1-800-430-5490
Burn Jel
Active ingredient
Lidocaine HCl 2.0%
Burn Jel
Purpose
External analgesic
Burn Jel
Uses
- temporarily relieves pain due to minor burns
Burn Jel
Warnings
For external use only
Do not use
- on large areas of the body, particularly over raw surfaces or blistered areas
Stop use and ask a doctor if
- the condition gets worse
- symptoms persist for more than 7 days
- condition clears up and recurs within a few days
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Burn Jel
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Other information
- store at room temperature
- do not use if opened or torn
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water ...
Burn Jel
Questions
1-800-430-5490
Povidone Iodine Swab
Active ingredient
Povidone-iodine solution USP, 10% (equivalent to 1% titratable iodine)
Povidone Iodine Swab
Purpose
First aid antiseptic
Povidone Iodine Swab
Uses
- first aid to help prevent the risk of infection in minor cuts, scrapes, and burns
Povidone Iodine Swab
Warnings
For external use only
Do not use
- over large areas of the body
- on individuals who are allergic or sensitive to iodine
Ask a doctor before use if you have
- deep or puncture wounds,
- animal bites
- serious burns
When using this product
- do not use longer than one wek unless directed by a doctor
Stop use and ask a doctor if
- conditions persists or gets worse
- irritation and redness develops
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Povidone Iodine Swab
Directions
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
Povidone Iodine Swab
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
Povidone Iodine Swab
Inactive ingredients
citric acid, disodium phosphate,nonoxynol-9, sodium hydroxide, water
Povidone Iodine Swab
Questions and comments
1-800-430-5490
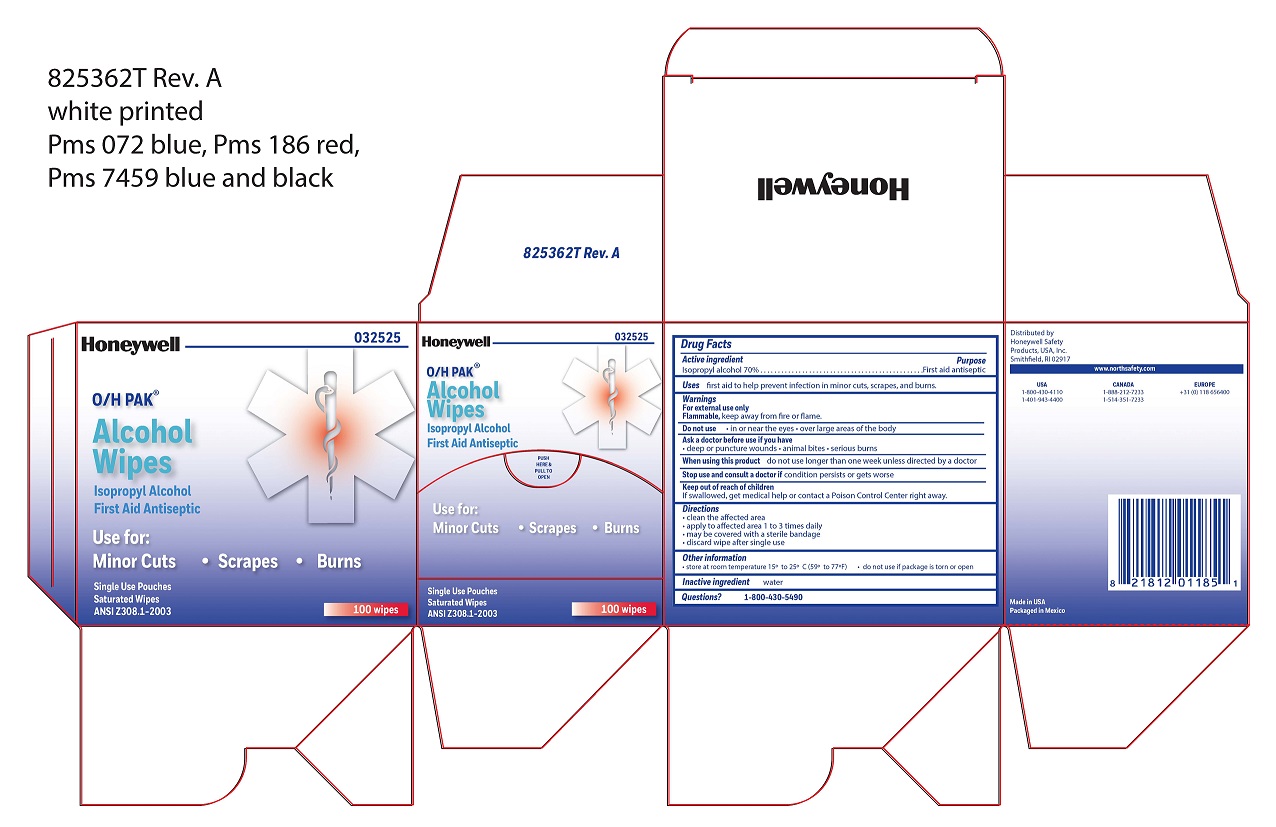
Alcohol Wipe
Active ingredient
Isopropyl alcohol 70%
Alcohol Wipe
Purpose
First aid antiseptic
Alcohol Wipe
Uses
- first aid to help prevent infection in minor cuts, scrapes, and burns
Alcohol Wipe
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burn
When using this product
- do not use longer than one week unless directed by a doctor
Stop use and consult a doctor
- if condition persists or gets worse
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Alcohol Wipe
Directions
- clean the affected area
- apply wipe to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard wipe after single use
Alcohol Wipe
Other information
store at room temperature 15
0 to 25
0 C (59
0 to 77
0F)
Alcohol Wipe
Inactive ingredient
water
Alcohol Wipe
Questions
1-800-430-5490
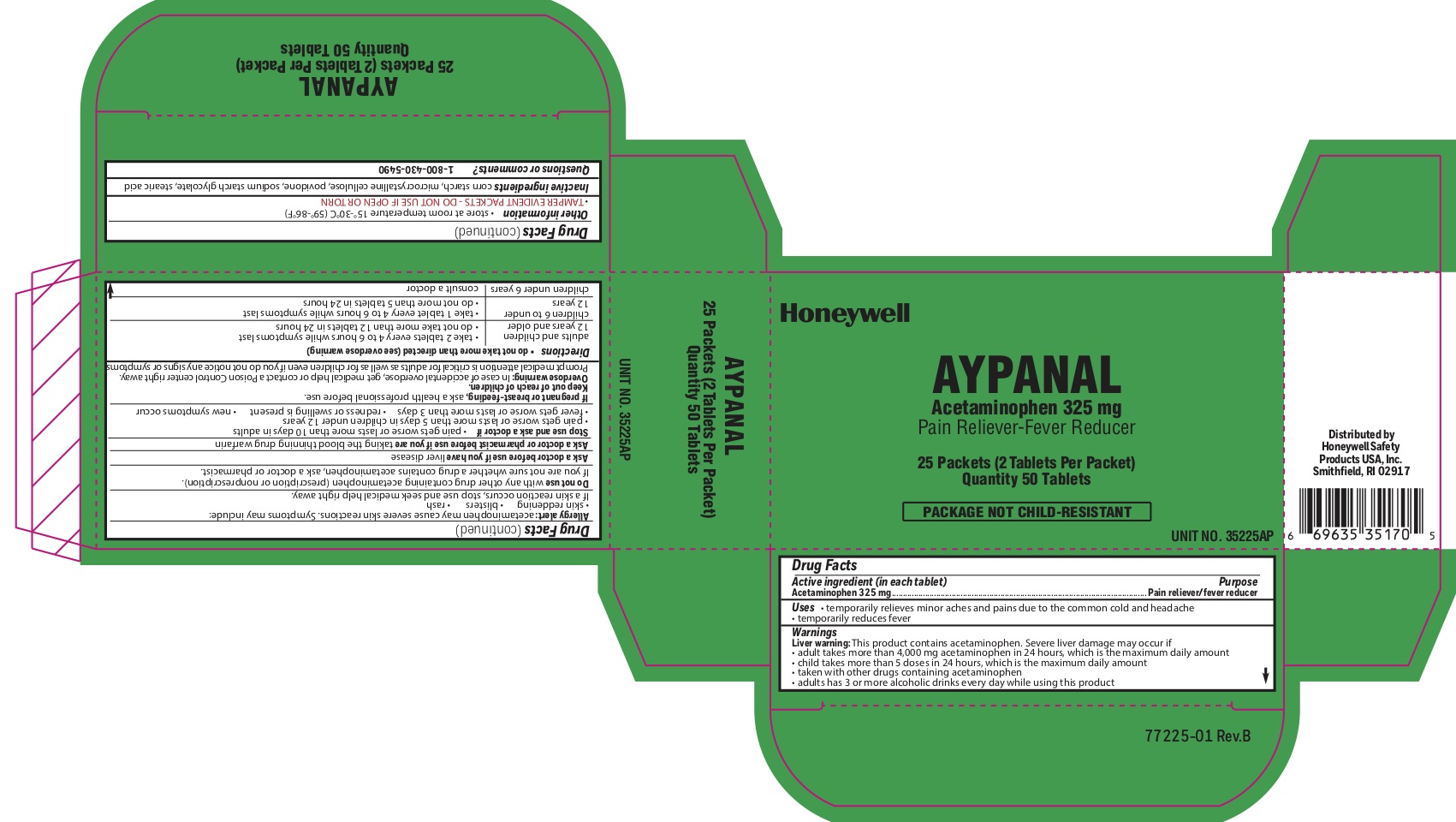
Aypanal
Active ingredient
Acetaminophen 325 mg
Aypanal
Purpose
Pain reliever/ fever reducer
Aypanaly
Uses
- temporarily relieves minor aches and pains due to the common cold and headache - temporarily reduces fever
Keep out of reach of children.
Keep out of reach of children.
Aypanal
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product:
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
- If a skin rash occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
Ask a doctor or pharmacist before use if
- you are taking the blood thinning drug warfarin
Stop using and ask a doctor if
- pain gets worse or lasts more than 10 days in adults
- pain gets worse or lasts more than 5 days in children under 12 years
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
If pregnant or breast-feeding
If pregnant or breast-feeding, ask a health professional before use.
Overdose warning
- In case of accidental overdose, get medical help or contact a Poison Control Center right away.
- Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Aypanal
Directions
do not take more than directed (see overdose warning)
adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than 12 tablets in 24 hours
children 6 to under 12 years of age
- take 1 tablet every 4-6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
children under 6 years consult a doctor
Aypanal
Other information
- store at room temperature 15
0 to 30
0 C (59
0 - 86
0 F)
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
Aypanal
Inactive ingredients
corn starch, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
Aypanal
Questions
1-800-430-5490
First Aid Burn Cream
Principal Display Panel
Sting Relief
Principal Display Panel
4187 Kit Label
010552-3305

Eyesaline
Principal Display Panel

Ammonia inhalent
Principal Display Panel
Burn Jel
Principal Display Panel

Povidone Iodine Swab
Principal Display Panel

Alcohol Wipe
Principal Display Panel
Aypanal
Principal Display Panel