ZO MEDICAL SURFATROL ASTRINGENT- aluminum acetate powder
ZO Skin Health, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
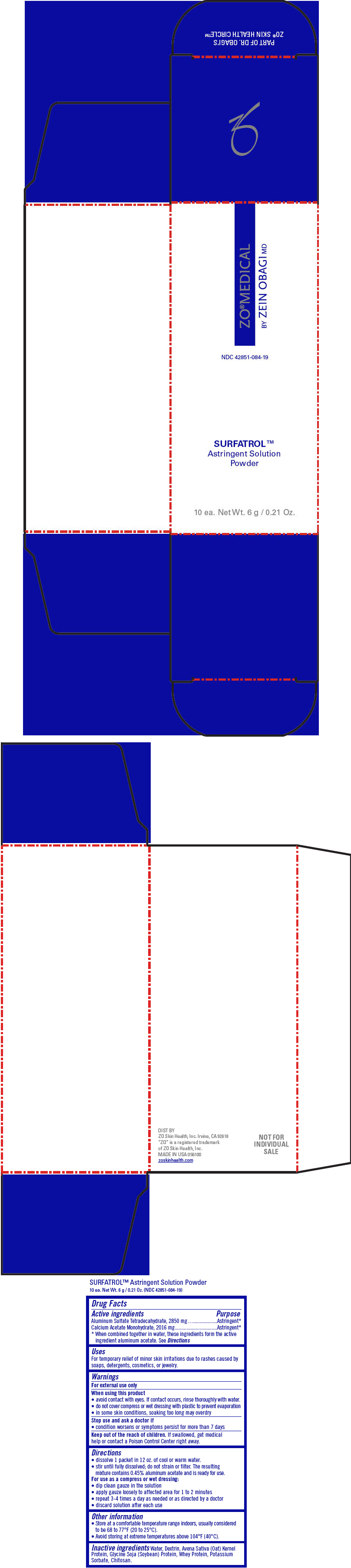
ZO ®MEDICAL SURFATROL™ Astringent Powder
Uses
For temporary relief of minor skin irritations due to rashes caused by soaps, detergents, cosmetics, or jewelry.
Warnings
For external use only
Directions
- dissolve 1 packet in 12 oz. of cool or warm water.
- stir until fully dissolved; do not strain or filter. The resulting mixture contains 0.45% aluminum acetate and is ready for use.
For use as a compress or wet dressing:
- dip clean gauze in the solution
- apply gauze loosely to affected area for 1 to 2 minutes
- repeat 3-4 times a day as needed or as directed by a doctor
- discard solution after each use
Other information
- Store at a comfortable temperature range indoors, usually considered to be 68 to 77°F (20 to 25°C).
- Avoid storing at extreme temperatures above 104°F (40°C).
| ZO MEDICAL SURFATROL ASTRINGENT
aluminum acetate powder |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ZO Skin Health, Inc. (826468527) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Paramount Cosmetics | 001321058 | manufacture(42851-084) | |
Revised: 8/2023
Document Id: 01e5d14d-888e-7764-e063-6294a90ada0b
Set id: 82f31fa8-960f-42c4-a0b1-9f91bad825b3
Version: 2
Effective Time: 20230801
ZO Skin Health, Inc.