Label: CHLORDIAZEPOXIDE HYDROCHLORIDE capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-0070-0, 54868-0070-2, 54868-2361-0, 54868-2361-3, view more54868-2361-4, 54868-2463-0, 54868-2463-1 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0555-0033, 0555-0158, 0555-0159
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated December 28, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Chlordiazepoxide hydrochloride is the prototype for the benzodiazepine compounds. It is a versatile therapeutic agent of proven value for the relief of anxiety. Chlordiazepoxide hydrochloride is among the safer of the effective psychopharmacologic compounds available, as demonstrated by extensive clinical evidence.
Chlordiazepoxide hydrochloride is 7-chloro-2- (methylamino)-5-phenyl-3H-1, 4-benzodiazepine 4-oxide hydrochloride. A white to practically white crystalline substance, it is soluble in water. It is unstable in solution and the powder must be protected from light. The structural formula is:
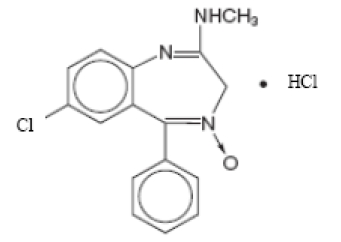
C16H14ClN3O • HCl M.W. 336.22
Each capsule, for oral administration, contains either 5 mg, 10 mg or 25 mg of chlordiazepoxide hydrochloride USP and has the following inactive ingredients: anhydrous lactose, D&C yellow no. 10, FD&C blue no. 1, FD&C blue no. 1 aluminum lake, gelatin, hydrogenated vegetable oil, microcrystalline cellulose, pharmaceutical glaze, and titanium dioxide. The 5 mg and 25 mg also contains D&C yellow no. 10 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, propylene glycol, and synthetic black iron oxide. In addition, the 5 mg contains D&C red no. 33 and the 10 mg also contains butyl paraben, edetate calcium disodium, dimethyl polysiloxane, ethylene glycol monoethyl ether, FD&C red no. 40, methyl paraben, propyl paraben, sodium, sodium lauryl sulfate, sodium propionate, and soya lecithin.
-
CLINICAL PHARMACOLOGY
Chlordiazepoxide HCl has antianxiety, sedative, appetite-stimulating and weak analgesic actions. The precise mechanism of action is not known. The drug blocks EEG arousal from stimulation of the brain stem reticular formation. It takes several hours for peak blood levels to be reached and the half-life of the drug is between 24 and 48 hours. After the drug is discontinued plasma levels decline slowly over a period of several days. Chlordiazepoxide is excreted in the urine, with 1% to 2% unchanged and 3% to 6% as conjugate.
Animal Pharmacology
The drug has been studied extensively in many species of animals and these studies are suggestive of action on the limbic system of the brain, which recent evidence indicates is involved in emotional responses.
Hostile monkeys were made tame by oral drug doses which did not cause sedation. Chlordiazepoxide HCl revealed a “taming” action with the elimination of fear and aggression. The taming effect of chlordiazepoxide HCl was further demonstrated in rats made vicious by lesions in the septal area of the brain. The drug dosage which effectively blocked the vicious reaction was well below the dose which caused sedation in these animals.
The LD50 of parenterally administered chlordiazepoxide HCl was determined in mice (72 hours) and rats (5 days), and calculated according to the method of Miller and Tainter, with the following results: mice, IV, 123 ± 12 mg/kg; mice, IM, 366 ± 7 mg/kg; rats, IV, 120 ± 7 mg/kg; rats, IM, > 160 mg/kg.
Effects on Reproduction
Reproduction studies in rats fed 10, 20 and 80 mg/kg daily and bred through one or two matings showed no congenital anomalies, nor were there adverse effects on lactation of the dams or growth of the newborn. However, in another study at 100 mg/kg daily there was noted a significant decrease in the fertilization rate and a marked decrease in the viability and body weight of off-spring which may be attributable to sedative activity, thus resulting in lack of interest in mating and lessened maternal nursing and care of the young. One neonate in each of the first and second matings in the rat reproduction study at the 100 mg/kg dose exhibited major skeletal defects. Further studies are in progress to determine the significance of these findings.
-
INDICATIONS AND USAGE
Chlordiazepoxide HCl capsules USP are indicated for the management of anxiety disorders or for the short term relief of symptoms of anxiety, withdrawal symptoms of acute alcoholism, and preoperative apprehension and anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The effectiveness of chlordiazepoxide HCl capsules USP in long term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
- CONTRAINDICATIONS
-
WARNINGS
Chlordiazepoxide HCl may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a vehicle or operating machinery. Similarly, it may impair mental alertness in children. The concomitant use of alcohol or other central nervous system depressants may have an additive effect. PATIENTS SHOULD BE WARNED ACCORDINGLY.
-
Usage in Pregnancy
An increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam and meprobamate) during the first trimester of pregnancy has been suggested in several studies. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug.
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE section).
-
PRECAUTIONS
In elderly and debilitated patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia or oversedation (10 mg or less per day initially, to be increased gradually as needed and tolerated). In general, the concomitant administration of chlordiazepoxide HCl and other psychotropic agents is not recommended. If such combination therapy seems indicated, careful consideration should be given to the pharmacology of the agents to be employed — particularly when the known potentiating compounds such as MAO inhibitors and phenothiazines are to be used. The usual precautions in treating patients with impaired renal or hepatic function should be observed.
Paradoxical reactions, e.g., excitement, stimulation and acute rage, have been reported in psychiatric patients and in hyperactive aggressive pediatric patients, and should be watched for during chlordiazepoxide HCl therapy. The usual precautions are indicated when chlordiazepoxide HCl is used in the treatment of anxiety states where there is any evidence of impending depression; it should be borne in mind that suicidal tendencies may be present and protective measures may be necessary. Although clinical studies have not established a cause and effect relationship, physicians should be aware that variable effects on blood coagulation have been reported very rarely in patients receiving oral anticoagulants and chlordiazepoxide HCl. In view of isolated reports associating chlordiazepoxide with exacerbation of porphyria, caution should be exercised in prescribing chlordiazepoxide to patients suffering from this disease.
Pediatric Use
Because of the varied response of pediatric patients to CNS-acting drugs, therapy should be initiated with the lowest dose and increased as required (see DOSAGE AND ADMINISTRATION). Since clinical experience with chlordiazepoxide HCl in pediatric patients under 6 years of age is limited, use in this age group is not recommended. Hyperactive aggressive pediatric patients should be monitored for paradoxical reactions to chlordiazepoxide HCl (see PRECAUTIONS).
Information for Patients
To assure the safe and effective use of benzodiazepines, patients should be informed that, since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug.
-
ADVERSE REACTIONS
The necessity of discontinuing therapy because of undesirable effects has been rare. Drowsiness, ataxia and confusion have been reported in some patients — particularly the elderly and debilitated. While these effects can be avoided in almost all instances by proper dosage adjustment, they have occasionally been observed at the lower dosage ranges. In a few instances syncope has been reported.
Other adverse reactions reported during therapy include isolated instances of skin eruptions, edema, minor menstrual irregularities, nausea and constipation, extrapyramidal symptoms, as well as increased and decreased libido. Such side effects have been infrequent, and are generally controlled with reduction of dosage. Changes in EEG patterns (low-voltage fast activity) have been observed in patients during and after chlordiazepoxide HCl treatment.
Blood dyscrasias (including agranulocytosis), jaundice and hepatic dysfunction have occasionally been reported during therapy. When chlordiazepoxide HCl treatment is protracted, periodic blood counts and liver function tests are advisable.
-
DRUG ABUSE AND DEPENDENCE
Chlordiazepoxide hydrochloride capsules are classified by the Drug Enforcement Administration as a Schedule IV controlled substance.
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting and sweating), have occurred following abrupt discontinuance of chlordiazepoxide. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving chlordiazepoxide or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
-
OVERDOSAGE
Manifestations of chlordiazepoxide overdosage includes somnolence, confusion, coma and diminished reflexes. Respiration, pulse and blood pressure should be monitored, as in all cases of drug overdosage, although, in general, these effects have been minimal following chlordiazepoxide HCl overdosage. General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. Hypotension may be combated by the use of norepinephrine or metaraminol. Dialysis is of limited value. There have been occasional reports of excitation in patients following chlordiazepoxide HCl overdosage; if this occurs barbiturates should not be used. As with the management of intentional overdosage with any drug, it should be borne in mind that multiple agents may have been ingested.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert, including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS, should be consulted prior to use.
-
DOSAGE AND ADMINISTRATION
Because of the wide range of clinical indications for chlordiazepoxide HCl, the optimum dosage varies with the diagnosis and response of the individual patient. The dosage, therefore, should be individualized for maximum beneficial effects.
ADULTS USUAL DAILY DOSE Relief of Mild and Moderate Anxiety Disorders and Symptoms of Anxiety
5 mg or 10 mg, 3 or 4 times daily Relief of Severe Anxiety Disorders and Symptoms of Anxiety
20 mg or 25 mg, 3 or 4 times daily Geriatric Patients, or in the presence of
debilitating disease.5 mg, 2 to 4 times daily Preoperative Apprehension and Anxiety
On days preceding surgery, 5 to 10 mg orally, 3 or 4 times daily. If used as preoperative medication, 50 to 100 mg IM* 1 hour prior to surgery.
PEDIATRIC PATIENTS USUAL DAILY DOSE Because of the varied response of pediatric patients to CNS-acting drugs, therapy should be initiated with the lowest dose and increased as required. Since clinical experience in pediatric patients under 6 years of age is limited, the use of the drug in this age group is not recommended.
5 mg, 2 to 4 times daily (may be increased in some pediatric patients to 10 mg, 2 to 3 times daily) For the relief of withdrawal symptoms of acute alcoholism, the parenteral form* is usually used initially. If the drug is administered orally, the suggested initial dose is 50 to 100 mg, to be followed by repeated doses as needed until agitation is controlled — up to 300 mg per day. Dosage should then be reduced to maintenance levels.
*See package insert for Injectable Chlordiazepoxide HCl.
-
HOW SUPPLIED
Chlordiazepoxide Hydrochoride Capsules USP, 5 mg are available as a two-piece hard gelatin capsule with an aqua green opaque cap and a yellow opaque body filled with white powder, imprinted in black ink barr 158, packaged in
Bottles of 30
NDC 54868-2463-1
Bottles of 100
NDC 54868-2463-0
Chlordiazepoxide Hydrochloride Capsules USP, 10 mg are available as a two-piece hard gelatin capsule with a black opaque cap and a green opaque body filled with white powder, imprinted in white ink barr 033, packaged in
Bottles of 20
NDC 54868-0070-2
Bottles of 30
NDC 54868-0070-0
Chlordiazepoxide Hydrochloride Capsules USP, 25 mg are available as a two-piece hard gelatin capsule with an aqua green opaque cap and a white opaque body filled with white powder, imprinted in black ink barr 159, available in
Bottles of 20
NDC 54868-2361-3
Bottles of 30
NDC 54868-2361-4
Bottles of 100
NDC 54868-2361-0
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] in a dry place.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. A 8/2011
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHLORDIAZEPOXIDE HYDROCHLORIDE
chlordiazepoxide hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-2463(NDC:0555-0158) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORDIAZEPOXIDE HYDROCHLORIDE (UNII: MFM6K1XWDK) (CHLORDIAZEPOXIDE - UNII:6RZ6XEZ3CR) CHLORDIAZEPOXIDE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) D&C RED NO. 33 (UNII: 9DBA0SBB0L) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color GREEN (aqua green) , YELLOW Score no score Shape CAPSULE Size 14mm Flavor Imprint Code barr;158 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-2463-0 100 in 1 BOTTLE, PLASTIC 2 NDC:54868-2463-1 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA084768 01/01/1993 CHLORDIAZEPOXIDE HYDROCHLORIDE
chlordiazepoxide hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-0070(NDC:0555-0033) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORDIAZEPOXIDE HYDROCHLORIDE (UNII: MFM6K1XWDK) (CHLORDIAZEPOXIDE - UNII:6RZ6XEZ3CR) CHLORDIAZEPOXIDE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) BUTYLPARABEN (UNII: 3QPI1U3FV8) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLENE GLYCOL MONOETHYL ETHER (UNII: IDK7C2HS09) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM PROPIONATE (UNII: DK6Y9P42IN) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BLACK, GREEN Score no score Shape CAPSULE Size 14mm Flavor Imprint Code barr;033 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-0070-0 30 in 1 BOTTLE, PLASTIC 2 NDC:54868-0070-2 20 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA083116 12/09/1994 CHLORDIAZEPOXIDE HYDROCHLORIDE
chlordiazepoxide hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-2361(NDC:0555-0159) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORDIAZEPOXIDE HYDROCHLORIDE (UNII: MFM6K1XWDK) (CHLORDIAZEPOXIDE - UNII:6RZ6XEZ3CR) CHLORDIAZEPOXIDE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color GREEN (aqua green) , WHITE Score no score Shape CAPSULE Size 14mm Flavor Imprint Code barr;159 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-2361-0 100 in 1 BOTTLE, PLASTIC 2 NDC:54868-2361-3 20 in 1 BOTTLE, PLASTIC 3 NDC:54868-2361-4 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA084769 03/24/1993 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel, repack






