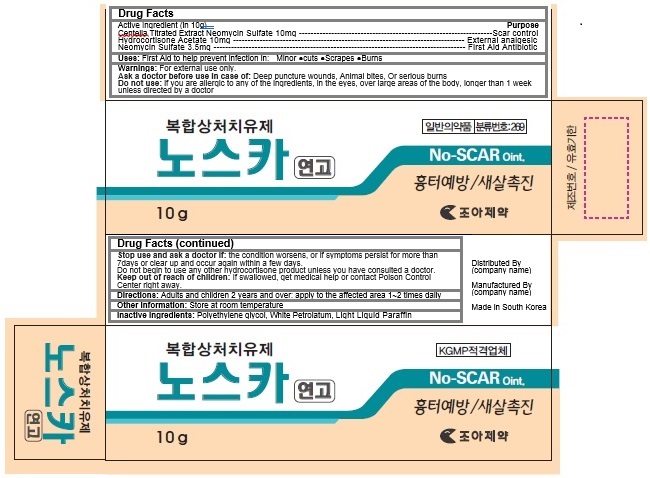
NO-SCAR OINT.- centella titrated extract neomycin sulfate, hydrocortisone acetate, neomycin sulfate ointment
Cho-A Pharm.Co.,Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
No-SCAR Oint.
Active Ingredients
Centella Titrated Extract 10mg
Hydrocortisone Acetate 10mg
Neomycin Sulfate 3.5 mg
Keep out of reach of children
If swallowed, get medical help or contact Poison Control Center right away.
Warnings
For external use only.
Ask a doctor before use in case of: Deep puncture wounds, Animal bites, Or serious burns
Do not use: if you are allergic to any of the ingredients, in the eyes, over large areas of the body, longer than 1 week unless directed by a doctor
Stop use and ask a doctor if: the condition worsens, or if symptoms persist for more than 7days or clear up and occur again within a few days.
Do not begin to use any other hydrocortisone product unless you have consulted a doctor.
| NO-SCAR OINT.
centella titrated extract neomycin sulfate, hydrocortisone acetate, neomycin sulfate ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cho-A Pharm.Co.,Ltd. (688056831) |
| Registrant - Cho-A Pharm.Co.,Ltd. (688056831) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cho-A Pharm.Co.,Ltd. | 688056831 | manufacture(58354-113) | |