Label: DUANE READE SUNSCREEN SPF 70- avobenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 67732-409-16 - Packager: DUANE READE INC.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 14, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
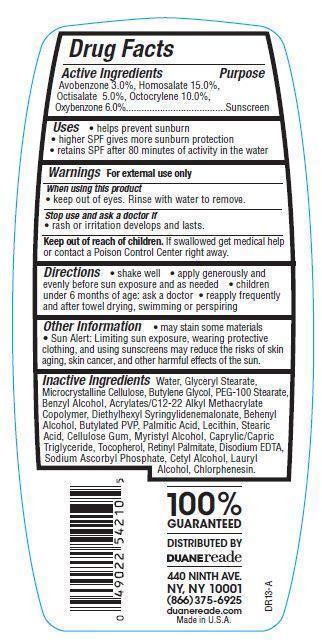
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
Water, Glyceryl Stearate, Microcrystalline Cellulose, Butylene Glcyol, PEG_100 Stearate, Benzyl Alcohol, Acrylates/C12-22 Alkyl Methcrylate Copolymer, Diethylhexyl Syringylidenemalonate, Behenyl Alcohol, Butylated PVP, Palmitic Acid, Lecithin, Stearic Acid, Cellulose Gum, Myristyl Alcohol, Caprylic/Capric Triglyceride, Tocopherol, Retinyl Palmitate, Disodium EDTA, Sodium Ascorbyl Phosphate, Cetyl Alcohol, Lauryl Alcohol, Chlorphenesin.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DUANE READE SUNSCREEN SPF 70
avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67732-409 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 15 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) STEARIC ACID (UNII: 4ELV7Z65AP) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PEG-100 STEARATE (UNII: YD01N1999R) BENZYL ALCOHOL (UNII: LKG8494WBH) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) DOCOSANOL (UNII: 9G1OE216XY) PALMITIC ACID (UNII: 2V16EO95H1) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) TOCOPHEROL (UNII: R0ZB2556P8) EDETATE DISODIUM (UNII: 7FLD91C86K) MYRISTYL ALCOHOL (UNII: V42034O9PU) TRICAPRYLIN (UNII: 6P92858988) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) CETYL ALCOHOL (UNII: 936JST6JCN) LAURYL ALCOHOL (UNII: 178A96NLP2) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67732-409-16 226 g in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/14/2012 Labeler - DUANE READE INC. (011988995)