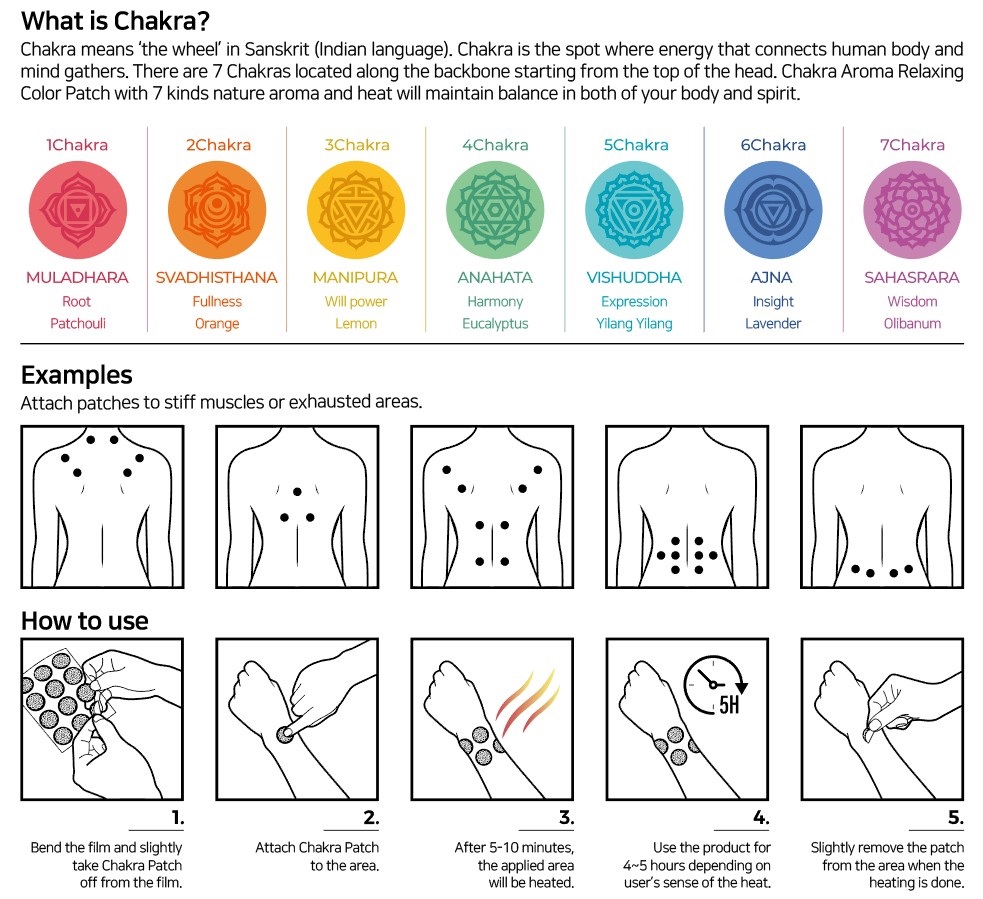
Label: CHAKRA AROMA RELAXING COLOR- capsicum oleoresin patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 72880-0100-1 - Packager: mooni studio
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 19, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Inactive ingredients
- Purposes
-
Warnings
- It is normal that the product heats the attached area, but if a rash appears on the skin, stop using the product immediately and go to see a doctor.
- Those who have sensitive skin or dermatitis should avoid long-hour usage(over 5 hours).
- Be careful using the product before or after sauna and hot massage when your skin may be more sensitive than the usual.
- If you feel itchy, remove the patch immediately and wash off the area with cold water.
- Do NOT use the product during pregnancy.
- Do NOT use the product to infants/children under 30 months.
- This product is NOT a medicine, but one of the manufactured goods
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- Package label
-
INGREDIENTS AND APPEARANCE
CHAKRA AROMA RELAXING COLOR
capsicum oleoresin patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72880-0100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSICUM OLEORESIN (UNII: UW86K581WY) (CAPSICUM OLEORESIN - UNII:UW86K581WY) CAPSAICIN 0.2 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SILVER (UNII: 3M4G523W1G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72880-0100-1 7 in 1 PACKAGE 01/01/2019 1 36 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/2019 Labeler - mooni studio (694861053) Establishment Name Address ID/FEI Business Operations mooni studio 694861053 label(72880-0100) , manufacture(72880-0100) , pack(72880-0100)