CVS BABY NIGHTTIME COLD AND COUGH- matricaria recutita, eupatorium perfoliatum flowering top, euphrasia stricta, gelsemium sempervirens root, and potassium iodide liquid
CVS Pharmacy
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
CVS Baby Nighttime Cold and Cough
| Active ingredients | Purpose |
|---|---|
| "HPUS" indicates that the active ingredients are in the official Homeopathic Pharmacopœia of the United States. | |
| Chamomilla 6X HPUS | occasional sleeplessness |
| Eupatorium Perfoliatum 6X HPUS | headache, cough, sneezing |
| Euphrasia Officinalis 6X HPUS | runny eyes and nose |
| Gelsemium Sempervirens 6X HPUS | sneezing with stuffy nose |
| Kali Iodatum 6X HPUS | headache, runny eyes and nose |
Uses
Temporarily relieves the symptoms of runny nose and eyes, cough, congestion, headache, sneezing and occasional sleeplessness due to common head colds in children.
Warnings
Do not use
- for persistent or chronic cough such as occurs with asthma, smoking or emphysema
- if cough is accompanied by excessive mucus, unless directed by a licensed health care professional
A persistent cough may be a sign of a serious condition.
Directions
- Measure only with the dosing syringe provided
- Do not use dosing syringe with other products
- On dosing syringe, mL = milliliter, tsp = teaspoon
| children under 6 months of age | consult a licensed health care professional before using this product |
| children 6 months to under 1 year of age | 2.5 mL or ½ teaspoon up to 4 times daily (every 6 hours) |
| children 1 to 3 years of age | 5 mL or 1 teaspoon up to 6 times daily (every 4 hours) |
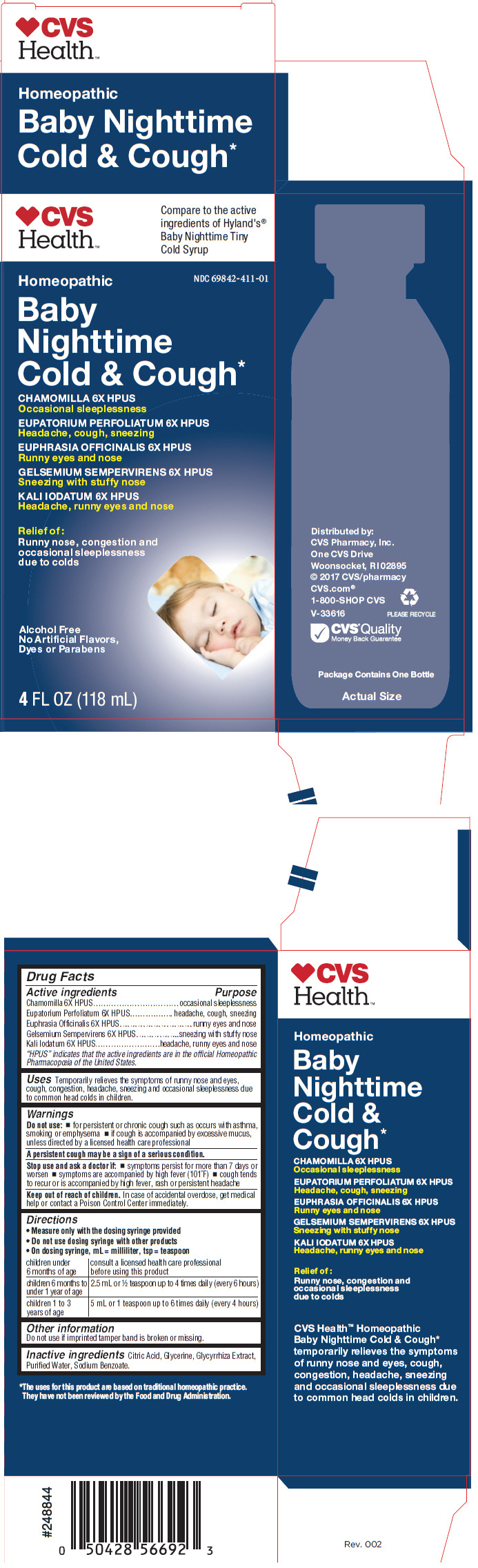
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
♥CVS
Health
™
Compare to the active
ingredients of Hyland's
®
Baby Nighttime Tiny
Cold Syrup
†
NDC 69842-411-01
Homeopathic
Baby
Nighttime
Cold & Cough
*
CHAMOMILLA 6X HPUS
Occasional sleeplessness
EUPATORIUM PERFOLIATUM 6X HPUS
Headache, cough, sneezing
EUPHRASIA OFFICINALIS 6X HPUS
Runny eyes and nose
GELSEMIUM SEMPERVIRENS 6X HPUS
Sneezing with stuffy nose
KALI IODATUM 6X HPUS
Headache, runny eyes and nose
Relief Of:
Runny nose, congestion and
occasional sleeplessness
due to colds
Alcohol Free
No Artificial Flavors,
Dyes or Parabens
4FL OZ (118 mL)
| CVS BABY NIGHTTIME COLD AND COUGH
matricaria recutita, eupatorium perfoliatum flowering top, euphrasia stricta, gelsemium sempervirens root, and potassium iodide liquid |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |