BRONCHITIS AND ASTHMA AIDE- aconitum napellus, calcium sulfide, spongia officinalis skeleton, roasted, tin tablet
Schwabe North America, Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
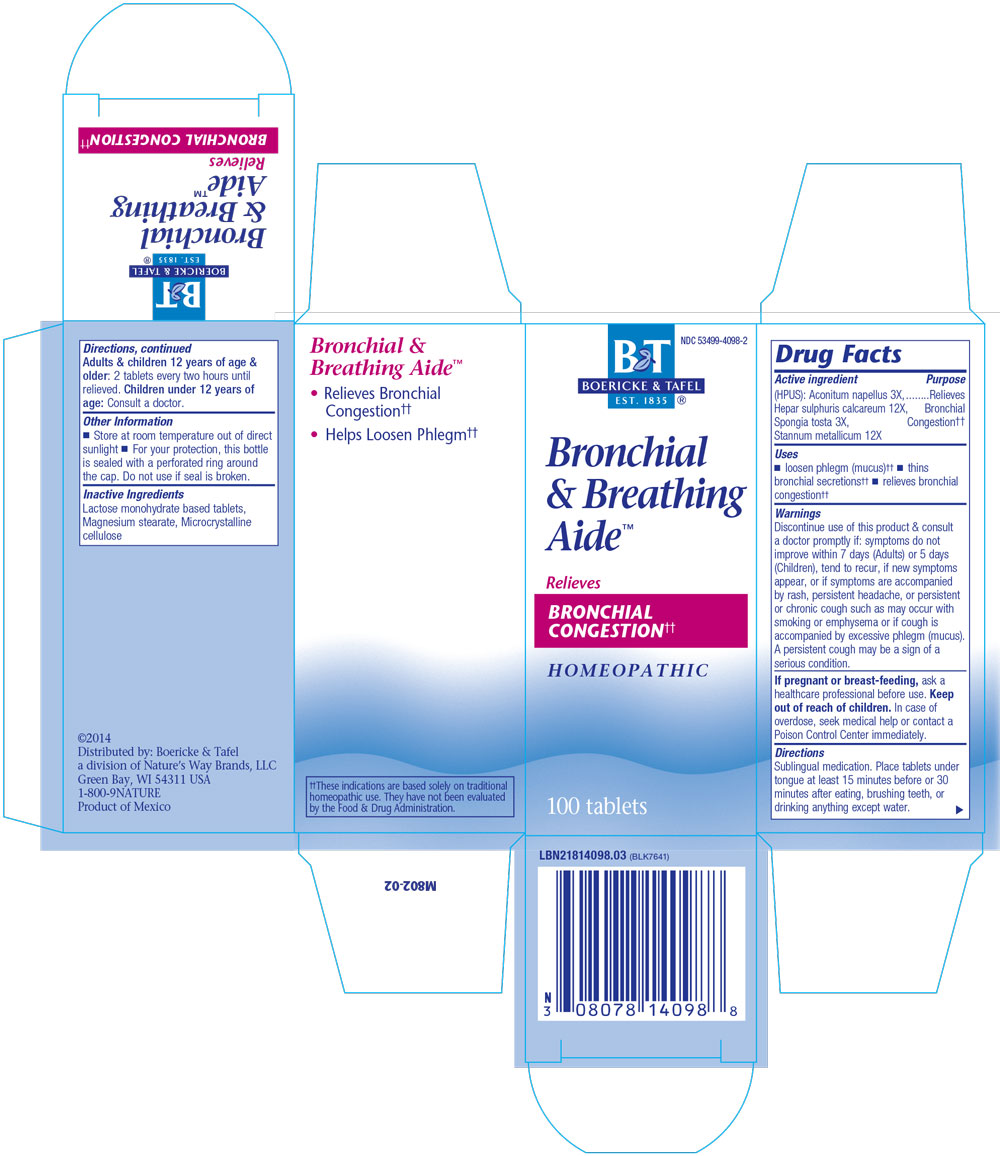
Bronchitis and Asthma Aide
ACTIVE INGREDIENTS:
Aconitum napellus 3X
Hepar sulphuris calcareum 12X
Spongia tosta 3X
Stannum metallicum 12X
PURPOSE:
Uses: Helps loosen phlegm (mucus) and thins bronchial secretions, relieves bronchial congestion
Directions: Sublingual medication.
Place tablets under tongue at least 15 minutes before or half hour after eating, brushing teeth, or drinking anything except water.
Adults and children 12 years of age and older: 2 tablets every two hours until relieved.
Children under 12 years of age: Consult a doctor.
INDICATIONS AND USAGE:
Helps loosen phlegm (mucus) and thins bronchial secretions, relieves bronchial congestion.
WARNINGS:
For Bronchitis
Discontinue use of this product and consult a doctor promptly if: symptoms do not improve within 7 days (for adults) or 5 days (for children), tend to recur, if new symptoms appear, or if symptoms are accompanied by rash, persistent headache, or persistent or chronic cough such as may occur with smoking or emphysema or if cough is accompanied by excessive phlegm (mucus).
A persistent cough may be a sign of a serious condition.
Pregnancy or Breast Feeding:
If pregnant or breast-feeding, ask a healthcare professional before use.
Ask the Doctor:
For Bronchitis
Discontinue use of this product and consult a doctor promptly if: symptoms do not improve within 7 days (for adults) or 5 days (for children), tend to recur, if new symptoms appear, or if symptoms are accompanied by rash, persistent headache, or persistent or chronic cough such as may occur with smoking or emphysema or if cough is accompanied by excessive phlegm (mucus).
A persistent cough may be a sign of a serious condition.
| BRONCHITIS AND ASTHMA AIDE
aconitum napellus, calcium sulfide, spongia officinalis skeleton, roasted, tin tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Schwabe North America, Inc (831153908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Schwabe Mexico SA DE CV | 812805901 | manufacture(53499-4098) | |