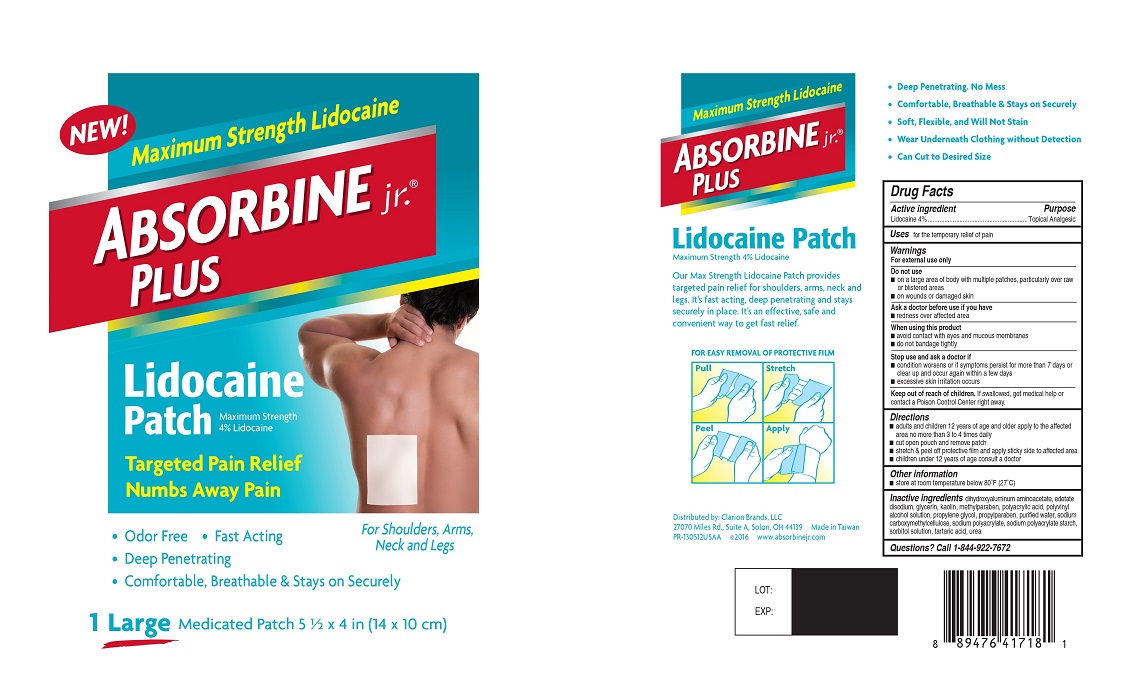
| Active ingredient | Purpose |
| Lidocaine 4% | Topical Analgesic |
Uses for the temporary relief of pain
Warnings
For external use only
Do not use
- on a large area of body with multiple patches, particularly over raw or blistered areas
- on wounds or damaged skin
Ask a doctor before use if you have
- redness over affected area
When using this product
- avoid contact with eyes and mucous membranes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days
- excessive skin irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years of age and older apply to the affected area no more than 3 to 4 times daily
- cut open pouch and remove patch
- stretch & peel off protective film and apply sticky side to affected area
- children under 12 years of age consult a doctor
Other information
- store at room temperature below 80ºF (27º C)
Inactive ingredients dihydroxyaluminum aminoacetate, edetate disodium, glycerin, kaolin, methylparaben, polyacrylic acid, polyvinyl alcohol solution, propylene glycol, propylparaben, purified water, sodium carboxymethylcellulose, sodium polyacrylate, sodium polyacrylate starch, sorbitol solution, tartaric acid, urea
Questions? Call 1-844-922-7672
Principal Display Panel
NEW!
Maximum Strength Lidocaine
ABSORBINE jr.® PLUS
Lidocaine Patch
Maximum Strength 4% Lidocaine
Targeted Pain Relief Numbs Away Pain
• Odor Free
• Fast Acting
• Deep Penetrating
• Comfortable, Breathable & Stays on Securely
For Shoulders, Arms, Neck and Legs
1 Large Medicated Patch 5 ½ x 4 in (14 x 10 cm)
Our Max Strength Lidocaine Patch provides targeted pain relief for shoulders, arms, neck and legs. It's fast acting, deep penetrating and stays securely in place. Its an effective, safe and convenient way to get fast relief.
FOR EASY REMOVAL OF PROTECTIVE FILM
Pull
Stretch
Peel
Apply
• Deep Penetrating. No mess
• Comfortable, Breathable & Stays on Securely
• Soft, Flexible, and Will Not Stain
• Wear Underneath Clothing without Detection
• Can Cut to Desired Size
Distributed by: Clarion Brands, LLC
27070 Miles Rd., Suite A, Solon, OH 44139 Made in Taiwan
PR-130512USAA ©2016 www.absorbinejr.com
LOT:
EXP: