RECOMBINATE- antihemophilic factor recombinant
Baxalta US Inc.
----------
RECOMBINATE [Antihemophilic Factor (Recombinant)]
Lyophilized Powder for Reconstitution for injection
Reconstitute with 10 mL of Sterile Water for Injection using double-ended needles
DESCRIPTION
RECOMBINATE [Antihemophilic Factor (Recombinant)] is a glycoprotein synthesized by a genetically engineered Chinese Hamster Ovary (CHO) cell line. In culture, the CHO cell line secretes recombinant Factor VIII (rFVIII) into the cell culture medium. The rFVIII is purified from the culture medium utilizing a series of chromatography columns. A key step in the purification process is an immunoaffinity chromatography methodology in which a purification matrix, prepared by immobilization of a monoclonal antibody directed to Factor VIII, is utilized to selectively isolate the rFVIII in the medium. The synthesized rFVIII produced by the CHO cells has the same biological effects as human Factor VIII. Structurally the protein has a similar combination of heterogenous heavy and light chains as found in human Factor VIII.
RECOMBINATE is formulated as a sterile, nonpyrogenic, off-white to faint yellow, lyophilized powder preparation of concentrated recombinant Factor VIII for intravenous injection. RECOMBINATE is available in single-dose vials, which contain nominally 250, 500 and 1000 International Units per vial. When reconstituted with the appropriate volume of diluent, the product contains the following stabilizers in maximum amounts: For 10 mL reconstitution volume: 12.5 mg/mL Albumin (Human), 0.20 mg/mL calcium, 1.5 mg/mL polyethylene glycol (3350), 180 mEq/L sodium, 55 mM histidine, 1.5 µg/Factor VIII International Unit (IU) polysorbate-80. Recombinant Von Willebrand Factor (rVWF) is coexpressed with the rFVIII and helps to stabilize it. The final product contains not more than 2 ng rVWF/IU rFVIII, which will not have any clinically relevant effect in patients with von Willebrand's disease. The product contains no preservative.
Manufacturing of RECOMBINATE is shared by Baxter Healthcare Corporation and Wyeth BioPharma. The recombinant Antihemophilic Factor Concentrate (For Further Manufacturing Use), is produced by Baxter Healthcare Corporation and Wyeth BioPharma (For Further Manufacturing Use) and subsequently formulated and packaged at Baxter Healthcare Corporation.
Each vial of RECOMBINATE is labeled with the Factor VIII activity expressed in IU per vial. Biological potency is determined by an in vitro assay which is referenced to the World Health Organization (WHO) International Standard for Factor VIII:C Concentrate.
CLINICAL PHARMACOLOGY
Factor VIII is the specific clotting factor deficient in patients with hemophilia A (classical hemophilia). Hemophilia A is a genetic bleeding disorder characterized by hemorrhages, which may occur spontaneously or after minor trauma. The administration of RECOMBINATE provides an increase in plasma levels of Factor VIII and can temporarily correct the coagulation defect in these patients. Pharmacokinetic studies on sixty-nine (69) patients revealed the circulating mean half-life for RECOMBINATE to be 14.6 ± 4.9 hours (n=67), which was not statistically significantly different from plasma-derived HEMOFIL M, [Antihemophilic Factor (Human), Method M, Monoclonal Purified]. The mean half-life of HEMOFIL M was 14.7 ± 5.1 hours (n=61). The actual baseline recovery observed with RECOMBINATE was 123.9 ± 47.7 IU/dL (n=23), which is significantly higher than the actual HEMOFIL M baseline recovery of 101.7 ± 31.6 IU/dL (n=61). However, the calculated ratio of actual to expected recovery with RECOMBINATE (121.2 ± 48.9%) is not different on average from HEMOFIL M (123.4 ± 16.4%).
The clinical study of RECOMBINATE in previously treated patients (individuals with hemophilia A who had been treated with plasma derived Factor VIII) was based on observations made on a study group of 69 patients. These individuals received cumulative amounts of Factor VIII ranging from 20,914 to 1,383,063 IU over the 48 month study. Patients were given a total of 17,700 infusions totaling 28,090,769 IU RECOMBINATE.
These patients were successfully treated for bleeding episodes on a demand basis and also for the prevention of bleeds (prophylaxis). Spontaneous bleeding episodes successfully managed include hemarthroses, soft tissue and muscle bleeds. Management of hemostasis was also evaluated in surgeries. A total of 24 procedures on 13 patients were performed during this study. These included minor (e.g. tooth extraction) and major (e.g. bilateral osteotomies, thoracotomy and liver transplant) procedures. Hemostasis was maintained perioperatively and postoperatively with individualized Factor VIII replacement.
A study of RECOMBINATE in previously untreated patients was also performed as part of an ongoing study. The study group was comprised of seventy-nine (79)1 patients , of whom seventy-six (76) had received at least one infusion of RECOMBINATE. To date, this cohort has been given 12,209 infusions totaling over 11,277,043 IU of RECOMBINATE. Hemostasis was appropriately managed in spontaneous bleeding episodes, intracranial hemorrhage and surgical procedures.
INDICATIONS and USAGE
The use of RECOMBINATE [Antihemophilic Factor (Recombinant)] is indicated in hemophilia A (classical hemophilia) for the prevention and control of hemorrhagic episodes.2 RECOMBINATE is also indicated in the perioperative management of patients with hemophilia A (classical hemophilia).
RECOMBINATE can be of therapeutic value in patients with acquired Factor VIII inhibitors not exceeding 10 Bethesda Units per mL.3 In clinical studies with RECOMBINATE, patients with inhibitors who were entered into the previously treated patient trial and those previously untreated children who have developed inhibitor activity on study, showed clinical hemostatic response when the titer of inhibitor was less than 10 Bethesda Units per mL. However, in such uses, the dosage of RECOMBINATE should be controlled by frequent laboratory determinations of circulating Factor VIII levels as well as the clinical status of the patient.
RECOMBINATE is not indicated in von Willebrand's disease.
CONTRAINDICATIONS
RECOMBINATE is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components, including bovine, mouse or hamster proteins.
WARNINGS
General
The clinical response to RECOMBINATE may vary. If bleeding is not controlled with the recommended dose, the plasma level of factor VIII should be determined and a sufficient dose of RECOMBINATE should be administered to achieve a satisfactory clinical response. If the patient's plasma factor VIII level fails to increase as expected or if bleeding is not controlled after the expected dose, the presence of an inhibitor (neutralizing antibodies) should be suspected and appropriate testing performed. (see PRECAUTIONS - Monitoring Laboratory Tests).
Anaphylaxis and Severe Hypersensitivity Reactions
Allergic type hypersensitivity reactions, including anaphylaxis, have been reported with RECOMBINATE and have been manifested as dizziness pruritus, rash, urticaria, flushing, angioedema/face swelling, laryngeal edema, dyspnea, pallor, pyrexia, nausea, paresthesia, hypotension, and loss of consciousness. Discontinue RECOMBINATE if symptoms occur and seek immediate emergency treatment. RECOMBINATE contains trace amounts of bovine proteins, mouse immunoglobulin G (MuIgG), and hamster (CHO) proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Neutralizing Antibodies
Patients treated with antihemophilic factor (AHF) products should be carefully monitored for the development of factor VIII inhibitors by appropriate clinical observations and laboratory tests. Inhibitors have been reported following administration of RECOMBINATE predominantly in previously untreated and minimally treated patients. The risk of developing inhibitors is highest during the first 20 exposure days. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an expected dose, an assay that measures factor VIII inhibitor concentration should be performed (see PRECAUTIONS - Monitoring Laboratory Tests).
PRECAUTIONS
General
Certain components used in the packaging of this product contain natural rubber latex.
Identification of the clotting defect as a Factor VIII deficiency is essential before the administration of RECOMBINATE [Antihemophilic Factor (Recombinant)] is initiated. No benefit may be expected from this product in treating other deficiencies.
Formation of Antibodies to Mouse, Hamster or Bovine Protein
As RECOMBINATE contains trace amounts of mouse protein (maximum of 0.1 ng/IU RECOMBINATE), hamster protein (maximum of 1.5 ng CHO protein/IU RECOMBINATE), and bovine protein (maximum of 1 ng BSA/IU RECOMBINATE), the remote possibility exists that patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Information for Patients
The patient and physician should discuss the risks and benefits of this product.
Allergic type hypersensitivity reactions have been observed with RECOMBINATE. Patients should be informed of the early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, symptoms of laryngeal edema, and anaphylaxis. Patients should be advised to discontinue use of the product and contact their physician if these symptoms occur.
Monitoring Laboratory Tests
- Monitor plasma factor VIII activity levels by the one-stage clotting assay to confirm the adequate factor VIII levels have been achieved and maintained, when clinically indicated. (seeDOSAGE AND ADMINISTRATION).
- Monitor for development of factor VIII inhibitors. Perform assay to determine if factor VIII inhibitor is present if expected factor VIII activity plasma levels are not attained, or if bleeding is not controlled with the expected dose of RECOMBINATE. Use Bethesda Units (BU) to titer inhibitors.
- If the inhibitor is less than 10 BU per mL, the administration of additional RECOMBINATE concentrate may neutralize the inhibitor, and may permit an appropriate hemostatic response.
- Adequate hemostasis may not be achieved if Inhibitor titers are above 10 BU per mL. The inhibitor titer may rise following RECOMBINATE infusion as a result of an anamnestic response to factor VIII. The treatment or prevention of bleeding in such patients requires the use of alternative therapeutic approaches and agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
RECOMBINATE was tested for mutagenicity at doses considerably exceeding plasma concentrations of Factor VIII in vitro and at doses up to ten times the expected maximum clinical dose in vivo, and did not cause reverse mutations, chromosomal aberrations, or an increase in micronuclei in bone marrow polychromatic erythrocytes. Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Pediatric Use
RECOMBINATE is appropriate for use in children of all ages, including the newborn. Safety and efficacy studies have been performed in both previously treated (n=23) and previously untreated (n=75) children. (See Clinical Pharmacology and Precautions).
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with RECOMBINATE. The safety of RECOMBINATE for use in pregnant women has not been established. It is not known whether RECOMBINATE can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Physicians should carefully consider the potential risks and benefits for each specific patient before prescribing RECOMBINATE. RECOMBINATE should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Adverse Reactions from Clinical Trials
During controlled clinical studies with RECOMBINATE enrolling 210 subjects, the most commonly reported adverse drug reactions were chills, flushing, rash and epistaxis.
| System Organ Class (SOC) | Preferred MedDRA Term | Number of Subjects | Percent of Evaluable Subjects* |
| GASTROINTESTINAL DISORDERS | Nausea | 1 | 0.48 |
| GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS |
Chills Fatigue Pyrexia |
3 1 1 |
1.43 0.48 0.48 |
| INFECTIONS AND INFESTATIONS | Ear infections | 1 | 0.48 |
| INVESTIGATIONS | Acoustic stimulation tests abnormal | 1 | 0.48 |
| MUSCULOSKELETAL AND CONNECTIVE TISSUES DISORDERS | Pain in extremity | 1 | 0.48 |
| NERVOUS SYSTEM DISORDERS |
Dizziness Tremors |
1 1 |
0.48 0.48 |
| RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS | Pharyngolaryngeal pain | 1 | 0.48 |
| SKIN AND SUBCUTANEOUS TISSUE DISORDERS |
Hyperhidrosis Pruritus Rash Rash maculopapular |
1 1 2 1 |
0.48 0.48 0.95 0.48 |
| VASCULAR DISORDERS |
Epistaxis Flushing Hematoma Hypotension Pallor Peripheral coldness |
1† 2 1 1 1 1 |
0.48 0.95 0.48 0.48 0.48 0.48 |
During the Previously Treated Patients (PTP) study, none of the 71 subjects developed de novo evidence of Factor VIII inhibitor. However, during the phase II/III portion of the study, 1 subject with a history of inhibitors exhibited inhibitor activity at 6 months (0.8 Bethesda Units [BU]), which resolved by 9 months. One other subject in this study had detectable Factor VIII inhibitor at baseline (1.26 BU) and exhibited an anamnestic response at 6 months (10.3 BU). During a prospective pharmaco-surveillance study of subjects who received batches of RECOMBINATE containing modestly increased Chinese Hamster Ovary (CHO) cell protein levels, none of the 34 treated subjects developed a Factor VIII inhibitor.
During the Previously Untreated Patients (PUP) study, 22 of the 73 evaluable subjects developed inhibitors to Factor VIII. Of these, 13 subjects displayed no detectable Factor VIII inhibitors at study exit.
Post-Marketing Adverse Reactions
In addition to the adverse reactions noted in clinical trials, the following adverse reactions have been reported in the post-marketing experience. These adverse reactions are listed by MedDRA (version 12.1) System Organ Class (SOC), then by MedDRA coding system Preferred Term in order of severity.
BLOOD AND LYMPHATIC SYSTEM DISORDERS: Factor VIII inhibition
CARDIAC DISORDERS: Tachycardia, Cyanosis
GASTROINTESTINAL DISORDERS: Vomiting, Abdominal pain
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS: Malaise, Injection site reactions, Chest pain, Chest discomfort
IMMUNE SYSTEM DISORDERS: Anaphylactic reaction, Hypersensitivity
NERVOUS SYSTEM DISORDERS: Loss of consciousness, Headache, Paresthesia
RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS: Dyspnea, Cough, Laryngeal edema
SKIN AND SUBCUTANEOUS TISSUE DISORDERS: Angioedema, Urticaria, Erythema
DOSAGE AND ADMINISTRATION
Each vial of RECOMBINATE is labeled with the Factor VIII activity expressed in IU per vial. This potency assignment is referenced to the World Health Organization International Standard for Factor VIII:C Concentrate and is evaluated by appropriate methodology to ensure accuracy of the results.
The expected in vivo peak increase in Factor VIII level expressed as IU/dL of plasma or % (percent) of normal can be estimated by multiplying the dose administered per kg body weight (IU/kg) by two. This calculation is based on the clinical findings of Abildgaard et al4 and is supported by the data generated by 419 clinical pharmacokinetic studies with RECOMBINATE in 67 patients over time. This pharmacokinetic data demonstrated a peak recovery point above the pre-infusion baseline of approximately 2.0 IU/dL per IU/kg body weight.
Examples (Assuming patient's baseline Factor VIII level is at <1%):
(1) A dose of 1750 IU RECOMBINATE administered to a 70 kg patient, i.e. 25 IU/kg (1750 IU/70 kg), should be expected to cause a peak post-infusion Factor VIII increase of 25 IU/kg x 2 (IU/dL)/(IU/kg) = 50 IU/dL (50% of normal).
(2) A peak level of 70% is required in a 40 kg child. In this situation, the dose would be 70 IU/dL/[2(IU/dL)/(IU/kg)] x 40 kg = 1400 IU.
Recommended Dosage Schedule
Physician supervision of the dosage is required. The following dosage schedule may be used as a guide.
| Hemorrhage | ||
| Degree of hemorrhage | Required peak post infusion Factor VIII activity in the blood (as % of normal or IU/dL plasma) | Frequency of Infusion |
| Early hemarthrosis or muscle bleed or oral bleed | 20-40 | Begin infusion every 12 to 24 hours for one-three days until the bleeding episode is resolved (as indicated by pain), or healing is achieved. |
| More extensive hemarthrosis, muscle bleed, or hematoma | 30-60 | Repeat infusion every 12 to 24 hours for (usually) three days or more until pain and disability are resolved. |
| Life threatening bleeds such as head injury, throat bleed, severe abdominal pain | 60-100 | Repeat infusion every 8 to 24 hours until threat is resolved |
| Surgery | ||
| Type of Operation | ||
| Minor surgery, including tooth extractions | 60-80 | A single infusion plus oral antifibrinolytic therapy within one hour is sufficient in approximately 70% of cases. |
| Major surgery | 80-100 (pre- and post-operative) | Repeat infusion every 8 to 24 hours depending on state of healing. |
If bleeding is not controlled with the recommended dose, the plasma level of Factor VIII should be determined and a sufficient dose of RECOMBINATE should be administered to achieve a satisfactory clinical response.
The careful control of the substitution therapy is especially important in cases of major surgery or life threatening hemorrhages. In presence of a low titer inhibitor, doses larger than those recommended may be necessary as per standard care.
Although dosage can be estimated by the calculations above, it is strongly recommended that whenever possible, appropriate laboratory tests including serial Factor VIII assays be performed on the patient's plasma at suitable intervals to assure that adequate Factor VIII levels have been reached and are maintained. Patients should be evaluated for the development of Factor VIII inhibitors, if the expected plasma Factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose.
Reconstitution: Use Aseptic Technique
- Bring RECOMBINATE, (dry factor concentrate) and Sterile Water for Injection, USP, (diluent) to room temperature.
- Remove caps from concentrate and diluent vials.
- Cleanse stoppers with germicidal solution and allow to dry prior to use. Place vials on a flat surface
- Remove protective covering from one end of double-ended needle and insert exposed needle through the center of the stopper.
- Remove protective covering from other end of double-ended needle. Invert diluent vial over the upright RECOMBINATE vial, then rapidly insert free end of the needle through the RECOMBINATE vial stopper at its center. The vacuum in the vial will draw in the diluent.
- Disconnect the two vials by removing needle from diluent vial stopper, then remove needle from RECOMBINATE vial. Swirl gently until RECOMBINATE is completely dissolved, otherwise active material will be removed by the filter needle. After reconstitution, the solution should be colorless to faint yellow, and substantially free from foreign particles.
NOTE: Do not refrigerate after reconstitution. (See Administration)
Administration: Use Aseptic Technique
RECOMBINATE is administered by intravenous (IV) injection after reconstitution.
Administer at room temperature.
RECOMBINATE should be administered not more than 3 hours after reconstitution.
Intravenous Syringe Injection
Parenteral drug products should be inspected for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be colorless to faint yellow in appearance. If not, do not use the solution and notify Baxter immediately.
Plastic syringes are recommended for use with this product since proteins such as RECOMBINATE tend to stick to the surface of glass syringes.
- Attach filter needle to a disposable syringe and draw back plunger to admit air into the syringe.
- Insert the needle into reconstituted RECOMBINATE .
- Inject air into vial and then withdraw the reconstituted material into the syringe.
- Remove and discard the filter needle from the syringe; attach a suitable needle and inject intravenously as instructed under Rate of Administration.
- If a patient is to receive more than one vial of RECOMBINATE, the contents of multiple vials may be drawn into the same syringe by drawing up each vial through a separate unused filter needle. Filter needles are intended to filter the contents of a single vial of RECOMBINATE only.
Rate of Administration
The rate of administration should be a rate that ensures the comfort of the patient. Preparations of RECOMBINATE can be administered at a rate of up to 10 mL per minute with no significant reactions when reconstituted with 10 mL sWFI.
The pulse rate should be determined before and during administration of RECOMBINATE. Should a significant increase in pulse rate occur, reducing the rate of administration or temporarily halting the injection usually allows the symptoms to disappear promptly.
HOW SUPPLIED
RECOMBINATE is available in three different strengths in single-dose vials. The strength is designated on the outer box and on the vial label using the following color codes:
| Color Code | Dosage Strength |
RECOMBINATE Supplied with 10 mL sWFI |
| Light blue bar | Low 220-400 IU per vial | NDC 0944-2831-15 |
| Light pink bar | Mid 401-800 IU per vial | NDC 0944-2832-15 |
| Light green bar | High 801-1240 IU per vial | NDC 0944-2833-15 |
RECOMBINATE is packaged with 10 mL of Sterile Water for Injection, USP, a double-ended needle, a filter needle, one physician insert and one patient insert.
STORAGE
RECOMBINATE can be refrigerated [2° - 8°C (36° - 46°F)] or stored at room temperature, not to exceed 30°C (86°F). Avoid freezing to prevent damage to the diluent vial. Do not use beyond the expiration date printed on the box.
CLINICAL STUDIES
Over the investigational period of the original safety and efficacy study of RECOMBINATE, none of the 69 subjects without an inhibitor at entry into the study, developed an inhibitor. In the previously untreated patient group there were 73 eligible subjects with Factor VIII levels less than or equal to 2% who received at least one RECOMBINATE treatment (median days 100, range 3-821) and who were tested for an inhibitor after treatment with RECOMBINATE. Of this group, 23 individuals (32%) developed a detectable inhibitor (median days on treatment at time of detection 10, range 3-69) and of these, 8 subjects (11%) showed a titer greater than 10 B.U.
REFERENCES
- Bray GL, Gomperts ED, Courter S, Gruppo R, et al: A Multicenter Study of Recombinant Factor VIII (Recombinate): Safety, Efficacy, and Inhibitor Risk in Previously Untreated Patients with Hemophilia A Blood 83:2428-2435, 1994
- White GC, McMillan CW, Kingdon HS, et al: Use of recombinant antihemophilic factor in the treatment of two patients with classic hemophilia. New Eng J Med 320:166-170, 1989
- Kessler CM: An Introduction to Factor VIII Inhibitors: The Detection and Quantitation. Am J Med 91 (Suppl 5A):1S-5S, 1991
- Abildgaard CF, Simone JV, Corrigan JJ, et al: Treatment of hemophilia with glycine-precipitated Factor VIII. New Eng J Med 275:471-475, 1966
To enroll in the confidential, industry-wide Patient Notification System, call 1-888-UPDATE U (1-888-873-2838).
Baxter, RECOMBINATE, and HEMOFIL, are trademarks of Baxter International Inc.
Manufactured by:
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Printed in USA
Revised Jan 2010
Patient Information
RECOMBINATE
[Antihemophilic Factor (Recombinant)]
This leaflet summarizes important information about RECOMBINATE. Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about RECOMBINATE. If you have any questions after reading this, ask your healthcare provider.
What is the most important information I need to know about RECOMBINATE [Antihemophilic Factor (Recombinant)]?
Do not attempt to do an infusion to yourself unless you have been taught how by your doctor or hemophilia center.
You must carefully follow your doctor's or other healthcare provider's instructions regarding the dose and schedule for infusing RECOMBINATE so that your treatment will work best for you.
What is RECOMBINATE [Antihemophilic Factor (Recombinant)]?
RECOMBINATE is a medicine used to replace a clotting factor (Factor VIII or antihemophilic factor) that is missing in people with hemophilia A (also called "classic" hemophilia). Hemophilia A is an inherited bleeding disorder that prevents blood from clotting normally.
RECOMBINATE is used to prevent and control bleeding in people with hemophilia A.
RECOMBINATE is not used to treat von Willebrand's Disease.
Who should not use RECOMBINATE [Antihemophilic Factor (Recombinant)]?
You should not use RECOMBINATE if you
- are allergic to mouse, hamster or bovine proteins.
- are allergic to any ingredients in RECOMBINATE.
Tell your healthcare provider if you are pregnant or breast-feeding because RECOMBINATE may not be right for you.
How should I use RECOMBINATE [Antihemophilic Factor (Recombinant)] ?
RECOMBINATE is given directly into the blood stream.
You may infuse RECOMBINATE at a hemophilia treatment center, at your healthcare provider's office or in your home. You should be trained on how to do infusions by your hemophilia treatment center or healthcare provider. Many people with hemophilia A learn to infuse their RECOMBINATE by themselves or with the help of a family member.
Your healthcare provider will tell you how much RECOMBINATE to use based on your weight, the severity of your hemophilia A, and where you are bleeding.
You may have to have blood tests done after getting RECOMBINATE to be sure that your blood level of Factor VIII is high enough to clot your blood.
Call your healthcare provider right away if your bleeding does not stop after taking RECOMBINATE.
What should I tell my healthcare provider before I use RECOMBINATE [Antihemophilic Factor (Recombinant)]?
You should tell your healthcare provider if you
- have or have had any medical problems.
- take any medicines, including non-prescription medicines and dietary supplements.
- have any allergies, including allergies to mouse, hamster or bovine proteins.
- are nursing.
- are pregnant.
- have been told that you have inhibitors to Factor VIII (because Factor VIII may not work for you).
What are the possible side effects of RECOMBINATE [Antihemophilic Factor (Recombinant)]?
You could have an allergic reaction to RECOMBINATE.
Call your healthcare provider right away and stop treatment if you get:
- Rash or hives
- itching
- tightness of the throat
- chest pain or tightness
- difficulty breathing
- light-headed, dizziness
- fainting
The most common side effects are chills, flushing, rash and nose bleeds. These are not all possible side effects with RECOMBINATE. You can ask your healthcare provider for information that is written for healthcare professionals. Tell your doctor about any side effect that bothers you or that does not go away.
What are the RECOMBINATE [Antihemophilic Factor (Recombinant)] dosage strengths?
RECOMBINATE comes in three different dosage strengths. The actual strength will be imprinted on the label and on the box. The three different strengths are coded, as follows:

Always check the potency printed on the label to make sure you are using the strength prescribed by your doctor. Always check the expiration date printed on the box. You should not use the product after the expiration date printed on the box.
How do I store RECOMBINATE [Antihemophilic Factor (Recombinant)]?
RECOMBINATE vials containing powdered product (without sterile diluent added) should be stored in a refrigerator (2° to 8°C [36° to 46°F]) or at room temperature (up to 30°C [86°F]).
If you choose to store RECOMBINATE at room temperature:
- it should remain at room temperature until infused.
- do not put room temperature product back in the refrigerator.
Store vials in their original box and protect them from extreme exposure to light.
Do not freeze.
Reconstituted product (after mixing dry product with wet diluent) must be used within 3 hours and cannot be stored or refrigerated. Any RECOMBINATE left in the vial at the end of your infusion should be discarded.
What else should I know about RECOMBINATE [Antihemophilic Factor (Recombinant)] and hemophilia A?
Your body may form inhibitors to Factor VIII. An inhibitor is part of the body's normal defense system. If you form inhibitors, it may stop RECOMBINATE from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to Factor VIII. Call your healthcare provider right away if your bleeding does not stop after taking RECOMBINATE.
Resources at Baxter available to the patients:
Contact Baxter to receive more product information:
Baxter Customer Service 1-800-423-2090
Baxter, RECOMBINATE, and HEMOFIL are trademarks of Baxter International Inc.
Manufactured by:
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Printed in USA Revised
Issued: Jan 2010
INSTRUCTIONS FOR USE
RECOMBINATE
Antihemophilic Factor (Recombinant)
(For intravenous use only)
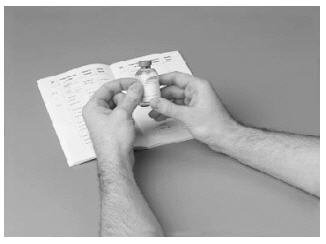
1. In a quiet place, prepare a clean surface. Let the vial with the FVIII concentrate and the Sterile Water for Injection (diluent) warm up to room temperature.
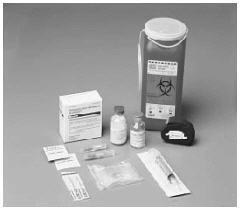
2. After washing your hands and putting on sterile gloves, remove caps from the concentrate and diluent vial to expose the centers of the rubber stoppers.
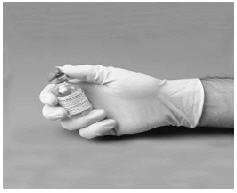
3. Disinfect the stoppers with an alcohol swab or other suitable solution suggested by your doctor or hemophilia center.
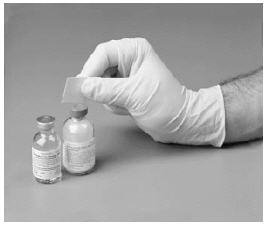
4. Remove the protective covering from one end of the double-ended transfer needle.
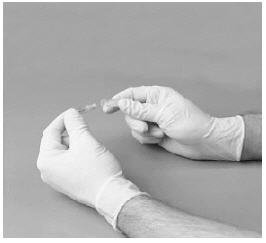
5. Insert the exposed short part of the needle through the diluent stopper.
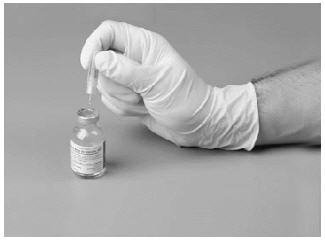
6. Remove the protective covering from the other end of the double-ended transfer needle.
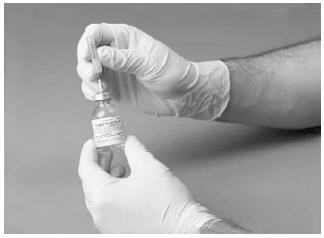
7. Insert the free end of the needle through the concentrate vial stopper at its center. The vacuum in the vial will draw in the diluent. Invert the diluent vial over the upright concentrate vial.
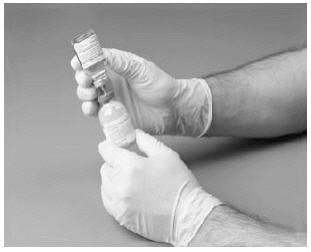
8. Disconnect the two vials by removing the needle from the diluent vials stopper, then remove the needle from the concentrate vials. Do not recap the needle! Place the needle in a hard-walled Sharps container for proper disposal.
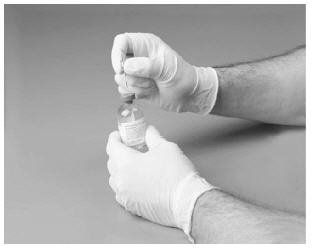
9. Gently roll the vial between palms until all material is dissolved. Do not shake. Check to make sure the product is completely dissolved.
10. Attach filter needle to a disposable syringe and draw back the plunger to allow air into the syringe. Insert the needle into the reconstituted FVIII concentrate. Inject air into the vial.
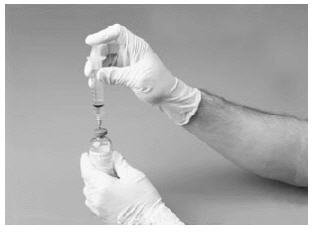
11. Withdraw the solution into the syringe.
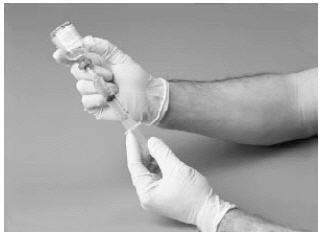
12. Prepare the injection site by wiping with an alcohol swab or other suitable solution suggested by your doctor or hemophilia center.
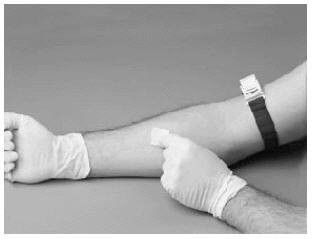
13. Remove and discard the filter needle from the syringe; attach a suitable needle. Use a winged infusion set if available. Do not infuse the product any faster than 10 mL per minute for RECOMBINATE dissolved with 10 mL of sWFI. After infusion, apply pressure with sterile gauze to the infusion site for 3 minutes. Do not recap the needle after infusion. Place it with the used syringe in a hard-walled Sharps container for proper disposal.
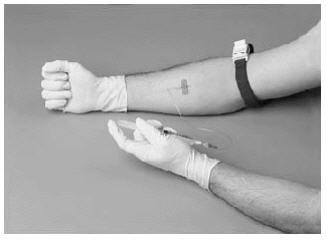
14. After infusion, remove peel-off label from the concentrate vial and place it in your factor log. Clean up any spilled blood with freshly prepared 10% bleach solution or soap and water.
IMPORTANT: Contact your doctor or local Hemophilia Treatment Center if you experience any problems.
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
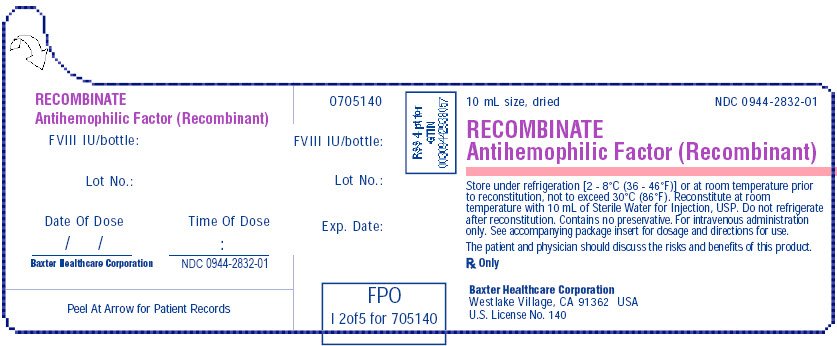
RECOMBINATE 500 IU vial label
10 mL size, dried
NDC 0944-2832-01
RECOMBINATE
Antihemophilic Factor (Recombinant)
Store under refrigeration [2° - 8°C (36 - 46°F) or at room temperature prior to reconstitution not to exceed 30°C (86°F). Reconstitute at room temperature with 10 mL of Sterile Water for Injection, USP. Do not refrigerate after reconstitution. Contains no preservative. For intravenous administration only. See accompanying package insert for dosage and directions for use.
The patient and physician should discuss the risks and benefits of the product.
Rx Only
Baxter Healthcare Corporation, Westlake Village, CA 91362 USA
U.S. License No 140

RECOMBINATE 500 IU unit carton
10 mL size, dried
NDC 0944-2832-15
RECOMBINATE
Antihemophilic Factor (Recombinant)
Baxter (logo)
For Intravenous Administration Only. See enclosed package insert for dosage and directions for use. Store under refrigeration [2° to 8°C (36° to 46°F)] or at room temperature, not to exceed 30°C (86°F). Avoid freezing to prevent damage to the diluent bottle.
During reconstitution, swirl bottle gently until all material is dissolved. Administer within three hours after reconstitution. Do not refrigerate after reconstitution.
The patient and physician should discuss the risks and benefits of this product.
Certain components used in the packaging of this product contain natural rubber latex.
Rx Only
Baxter and Recombinate are trademarks of Baxter International Inc.
Baxter is registered in the U.S. Patent and Trademark office.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140

10 mL Sterile Water for Injection vial label
1A7139
DIN 02214733
NDC 0338-0764-61
10 mL Single-Dose Container Nonpyrogenic
Sterile Water for Injection, USP for reconstitution of accompanying product
Do not use unless clear. No antimicrobial agent or other substances has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion. Rx Only. This Product Contains Dry Natural Rubber.
Baxter
Manufactured by Baxter Healthcare Corporation Deerfield, IL 60015 USA
Imported by Baxter Corporation Toronto, Ontario
| RECOMBINATE
antihemophilic factor recombinant kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| RECOMBINATE
antihemophilic factor recombinant kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| RECOMBINATE
antihemophilic factor recombinant kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxalta US Inc. (079887619) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Corporation | 001728059 | MANUFACTURE(0944-2831, 0944-2832, 0944-2833) , LABEL(0944-2831, 0944-2832, 0944-2833) | |