COLGATE SENSITIVE PREVENT AND REPAIR- sodium fluoride and potassium nitrate paste, dentifrice
Colgate-Palmolive Company
----------
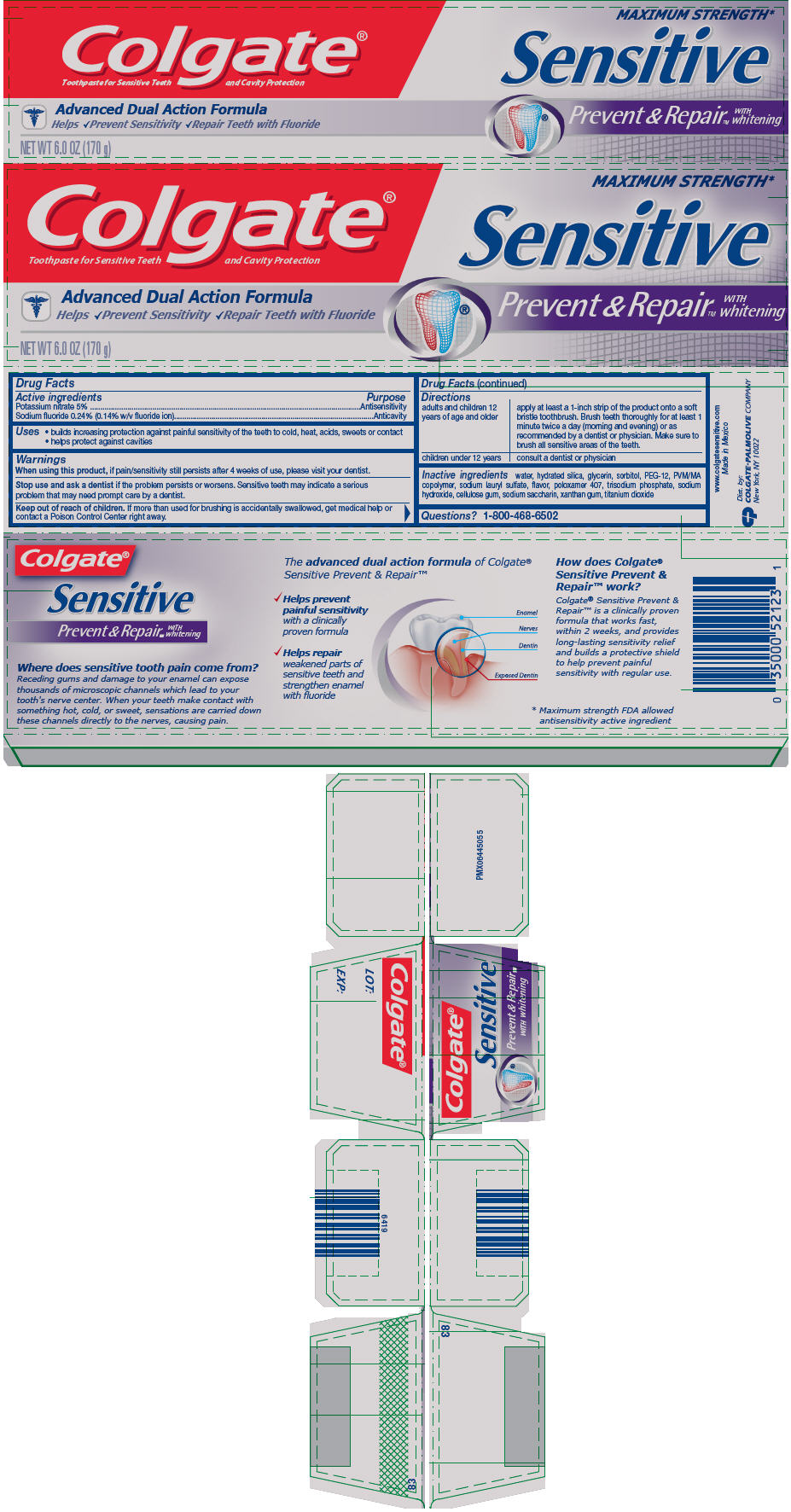
Colgate® Sensitive Prevent & Repair™ with Whitening Toothpaste
Uses
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
- helps protect against cavities
Warnings
When using this product, if pain/sensitivity still persists after 4 weeks of use, please visit your dentist.
Directions
| adults and children 12 years of age and older | apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist or physician. Make sure to brush all sensitive areas of the teeth. |
| children under 12 years | consult a dentist or physician |
| COLGATE SENSITIVE PREVENT AND REPAIR
sodium fluoride and potassium nitrate paste, dentifrice |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Colgate-Palmolive Company (001344381) |
Revised: 12/2019
Document Id: 58cf5b6d-c1d9-456e-b909-a04828a3407d
Set id: 811373b0-fae5-47db-9b4a-33a216fe7bf6
Version: 5
Effective Time: 20191217
Colgate-Palmolive Company