Label: ELZONRIS- tagraxofusp injection, solution
- NDC Code(s): 72187-0401-1
- Packager: Stemline Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 31, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use
ELZONRIS®safely and effectively. See full prescribing information for
ELZONRIS.
ELZONRIS (tagraxofusp-erzs) injection, for intravenous use
Initial U.S. Approval: 2018WARNING: CAPILLARY LEAK SYNDROME
See full prescribing information for complete boxed warning.
Capillary Leak Syndrome (CLS), which may be life-threatening or fatal if not properly managed, can occur in patients receiving ELZONRIS. (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ELZONRIS is a CD123-directed cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and in pediatric patients 2 years and older (1)
DOSAGE AND ADMINISTRATION
- Premedicate with an H1-histamine antagonist, acetaminophen, corticosteroid and H2-histamine antagonist prior to each ELZONRIS infusion. (2.1)
- Administer ELZONRIS intravenously at 12 mcg/kg over 15 minutes once daily on days 1 to 5 of a 21-day cycle. (2.1)
- Administer the first cycle of ELZONRIS in the inpatient setting. Subsequent cycles may be administered in the inpatient or appropriate outpatient setting. (2.1)
- Additional important preparation and administration information is in full prescribing information. See full prescribing information for instructions on preparation and administration. (2.3, 2.4)
DOSAGE FORMS AND STRENGTHS
- Injection: 1,000 mcg in 1 mL in a single-dose vial (3)
CONTRAINDICATIONS
- None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 30%) are capillary leak syndrome, nausea, fatigue, pyrexia, peripheral edema, and weight increase. Most common laboratory abnormalities (incidence ≥ 50%) are decreases in albumin, platelets, hemoglobin, calcium, and sodium, and increases in glucose, ALT and AST. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Stemline Therapeutics, Inc. at 1-877-332-7961 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Lactation: Advise women not to breastfeed (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CAPILLARY LEAK SYNDROME
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications
2.3 Preparation for Administration
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Capillary Leak Syndrome
5.2 Hypersensitivity Reactions
5.3 Hepatotoxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 First-Line Treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
14.2 Relapsed or Refractory Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CAPILLARY LEAK SYNDROME
Capillary Leak Syndrome (CLS) which may be life-threatening or fatal, can occur in patients receiving ELZONRIS. Monitor for signs and symptoms of CLS and take actions as recommended [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
- Administer ELZONRIS at 12 mcg/kg intravenously over 15 minutes once daily on days 1 to 5 of a 21-day cycle. The dosing period may be extended for dose delays up to day 10 of the cycle. Continue treatment with ELZONRIS until disease progression or unacceptable toxicity. The dose is calculated based on the patient’s actual weight.
- Prior to the first dose of the first cycle, ensure serum albumin is greater than or equal to 3.2 g/dL before administering ELZONRIS.
- Premedicate patients with an H1-histamine antagonist (e.g., diphenhydramine hydrochloride), H2-histamine antagonist (e.g., famotidine), corticosteroid (e.g., 50 mg intravenous methylprednisolone or equivalent) and acetaminophen (or paracetamol) approximately 60 minutes prior to each ELZONRIS infusion.
- Administer Cycle 1 of ELZONRIS in the inpatient setting with patient observation through at least 24 hours after the last infusion.
- Administer subsequent cycles of ELZONRIS in the inpatient setting or in a suitable outpatient ambulatory care setting that is equipped with appropriate monitoring for patients with hematopoietic malignancies undergoing treatment. Observe patients for a minimum of 4 hours following each infusion.
2.2 Dosage Modifications
Monitor vital signs and check albumin, transaminases, and creatinine prior to preparing each dose of ELZONRIS. See Table 1 for recommended dose modifications and Table 2 for CLS management guidelines.
Table 1. Recommended ELZONRIS Dosage Modifications Parameter Severity Criteria Dosage Modification Serum albumin Serum albumin < 3.5 g/dL or reduced ≥ 0.5 g/dL from value measured prior to initiation of the current cycle See CLS Management Guidelines (Table 2) Body weight Body weight increase ≥ 1.5 kg over pretreatment weight on prior treatment day See CLS Management Guidelines (Table 2) Aspartate
aminotransferase (AST)
or alanine
aminotransferase (ALT)ALT or AST increase > 5 times the upper limit of normal Withhold ELZONRIS until transaminase elevations are ≤ 2.5 times the upper limit of normal. Serum creatinine Serum creatinine > 1.8 mg/dL (159 micromol/L) or creatinine clearance < 60 mL/minute Withhold ELZONRIS until serum creatinine resolves to ≤ 1.8 mg/dL (159 micromol/L) or creatinine clearance ≥ 60 mL/minute. Systolic blood pressure Systolic blood pressure ≥ 160 mmHg or ≤ 80 mmHg Withhold ELZONRIS until systolic blood pressure is < 160 mmHg or > 80 mmHg. Heart rate Heart rate ≥ 130 bpm or ≤ 40 bpm Withhold ELZONRIS until heart rate is < 130 bpm or > 40 bpm. Body temperature Body temperature ≥ 38°C Withhold ELZONRIS until body temperature is < 38°C. Hypersensitivity reactions Mild or moderate Withhold ELZONRIS until resolution of any mild or moderate hypersensitivity reaction. Resume ELZONRIS at the same infusion rate. Severe or life-threatening Discontinue ELZONRIS permanently. Table 2. CLS Management Guidelines 1If ELZONRIS dose is held:
- ELZONRIS administration may resume in the same cycle if all CLS signs/symptoms have resolved and the patient did not require measures to treat hemodynamic instability
- ELZONRIS administration should be held for the remainder of the cycle if CLS signs/symptoms have not resolved or the patient required measures to treat hemodynamic instability (e.g., required administration of intravenous fluids and/or vasopressors to treat hypotension) (even if resolved), and
- ELZONRIS administration may only resume in the next cycle if all CLS signs/symptoms have resolved, and the patient is hemodynamically stable.
Time of Presentation CLS
Sign/SymptomRecommended
ActionELZONRIS
Dosing
ManagementPrior to first dose of ELZONRIS in cycle 1 Serum albumin < 3.2 g/dL Administer ELZONRIS when serum albumin ≥ 3.2 g/dL. During ELZONRIS dosing Serum albumin < 3.5 g/dL Administer 25g intravenous albumin (q12h or more frequently as practical) until serum albumin is ≥ 3.5 g/dL AND not more than 0.5 g/dL lower than the value measured prior to dosing initiation of the current cycle. Interrupt ELZONRIS dosing until the relevant CLS sign/symptom has resolved1. Serum albumin reduced by ≥ 0.5 g/dL from the albumin value measured prior to ELZONRIS dosing initiation of the current cycle A predose body weight that is increased by ≥ 1.5 kg over the previous day’s predose weight Administer 25g intravenous albumin (q12h or more frequently as practical), and manage fluid status as indicated clinically (e.g., generally with intravenous fluids and vasopressors if hypotensive and with diuretics if normotensive or hypertensive), until body weight increase has resolved (i.e. the increase is no longer ≥ 1.5 kg greater than the previous day’s predose weight). Edema, fluid overload and/or hypotension Administer 25g intravenous albumin (q12h, or more frequently as practical) until serum albumin is ≥ 3.5 g/dL.
Administer 1 mg/kg of methylprednisolone (or an equivalent) per day, until resolution of CLS sign/symptom or as indicated clinically.
Aggressive management of fluid status and hypotension if present, which could include intravenous fluids and/or diuretics or other blood pressure management, until resolution of CLS sign/symptom or as clinically indicated.2.3 Preparation for Administration
Assure the following components required for dose preparation and administration are available prior to thawing ELZONRIS:
-
- One infusion syringe pump
- One empty 10 mL sterile vial
- 0.9% Sodium Chloride Injection, USP
- Three 10 mL sterile syringes
- One 1 mL sterile syringe
- One mini-bifuse Y-connector
- Microbore tubing
- One 0.2 micron polyethersulfone in-line filter
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Thawed ELZONRIS appearance should be a clear, colorless liquid that may contain a few white to translucent particles.
- Prior to dose preparation thaw at room temperature, between 15°C and 25°C (59°F and 77°F), for 15 to 30 minutes in original carton, and verify thaw visually. Thawed vials may be held at room temperature for approximately 1 hour prior to dosage preparation. Do not force thaw. Do not refreeze vial once thawed.
- Use aseptic technique for preparation of the ELZONRIS dose.
- A 2-step process is required for preparation of the final ELZONRIS dose:
- -
- Step 1 - Prepare 10 mL of 100 mcg/mL ELZONRIS
- -
- Using a sterile 10 mL syringe, transfer 9 mL of 0.9% Sodium Chloride Injection, USP to an empty sterile 10 mL vial.
- -
- Gently swirl the ELZONRIS vial to mix the contents, remove the cap, and using a sterile 1 mL syringe, withdraw 1 mL of thawed ELZONRIS from the product vial.
- -
- Transfer the 1 mL of ELZONRIS into the 10 mL vial containing the 0.9% Sodium Chloride Injection, USP. Gently invert the vial at least 3 times to mix the contents. Do not shake vigorously.
- -
- Following dilution the final concentration of ELZONRIS is 100 mcg/mL.
- -
- Step 2 – Prepare the ELZONRIS infusion set.
- -
- Calculate the required volume of diluted ELZONRIS (100 mcg/mL) according to patient’s weight.
- -
- Draw up the required volume into a new syringe (if more than 10 mL of diluted ELZONRIS (100 mcg/mL) is required for the calculated patient dose, repeat step 1 with a second vial of ELZONRIS). Label the ELZONRIS syringe.
- -
- Prepare a separate syringe with at least 3 mL of 0.9% Sodium Chloride Injection, USP to be used to flush the administration set once the ELZONRIS dose is delivered.
- -
- Label the 0.9% Sodium Chloride Injection, USP flush syringe.
- -
- Connect the 0.9% Sodium Chloride Injection, USP flush syringe to one arm of the Y-connector and ensure the clamp is closed.
- -
- Connect the product syringe to the other arm of the Y-connector and ensure the clamp is closed.
- -
- Connect the terminal end of the Y-connector to the microbore tubing.
- -
- Remove the cap from the supply side of the 0.2 micron filter and attach it to the terminal end of the microbore tubing.
- -
- Unclamp the arm of the Y-connector connected to the 0.9% Sodium Chloride Injection, USP flush syringe. Prime the Y-connector up to the intersection (do not prime the full infusion set with 0.9% Sodium Chloride Injection, USP). Re-clamp the Y-connector line on the 0.9% Sodium Chloride Injection, USP flush arm.
- -
- Remove the cap on the terminal end of the 0.2 micron filter and set it aside. Unclamp the arm of the Y-connector connected to the product syringe, and prime the entire infusion set, including the filter. Recap the filter, and re-clamp the Y-connector line on the product side. The infusion set is now ready for delivery for dose administration.
- Administer ELZONRIS within 4 hours. During this 4-hour window, the prepared dose should remain at room temperature.
- Do not reuse excess ELZONRIS. Any excess material should be thrown away immediately following infusion.
2.4 Administration
- Establish venous access and maintain with sterile 0.9% Sodium Chloride Injection, USP.
- Administer the prepared ELZONRIS dose via infusion syringe pump over 15 minutes. The total infusion time will be controlled using a syringe pump to deliver the entire dose and the 0.9% Sodium Chloride Injection, USP flush over 15 minutes.
- Insert the ELZONRIS syringe into the syringe pump, open the clamp on the ELZONRIS side of the Y-connector and deliver the prepared ELZONRIS dose.
- Once the ELZONRIS syringe has been emptied, remove it from the pump and place the 0.9% Sodium Chloride Injection, USP flush syringe in the syringe pump.
- Open the clamp on the 0.9% Sodium Chloride Injection, USP flush side of the Y-connector and resume infusion via the syringe pump at the pre-specified flow to push remaining ELZONRIS dose out of the infusion line to complete delivery.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Capillary Leak Syndrome
Capillary leak syndrome (CLS), including life-threatening and fatal cases, has been reported among patients treated with ELZONRIS. In patients receiving ELZONRIS in clinical trials, the overall incidence of CLS was 53% (65/122), including Grade 1 or 2 in 43% (52/122) of patients, Grade 3 in 7% (8/122) of patients, Grade 4 in 1% (1/122) of patients, and four fatalities (3%) [see Adverse Reactions (6.1)]. The median time to onset was 4 days (range - 1 to 46 days), and all but 5 patients experienced an event in Cycle 1.
Before initiating therapy with ELZONRIS, ensure that the patient has adequate cardiac function and serum albumin is greater than or equal to 3.2 g/dL. During treatment with ELZONRIS, monitor serum albumin levels prior to the initiation of each dose of ELZONRIS and as indicated clinically thereafter, and assess patients for other signs or symptoms of CLS, including weight gain, new onset or worsening edema, including pulmonary edema, hypotension or hemodynamic instability [see Dosage and Administration (2.2)].
5.2 Hypersensitivity Reactions
ELZONRIS can cause severe hypersensitivity reactions. In patients receiving ELZONRIS in clinical trials, hypersensitivity reactions were reported in 43% (53/122) of patients treated with ELZONRIS and were Grade ≥ 3 in 7% (9/122) [see Adverse Reactions (6.1)]. Manifestations of hypersensitivity reported in ≥ 5% of patients include rash, pruritus, and stomatitis. Monitor patients for hypersensitivity reactions during treatment with ELZONRIS. Interrupt ELZONRIS infusion and provide supportive care as needed if a hypersensitivity reaction should occur [see Dosage and Administration (2.2)].
5.3 Hepatotoxicity
Treatment with ELZONRIS was associated with elevations in liver enzymes. In patients receiving ELZONRIS in clinical trials, elevations in ALT occurred in 79% (96/122) and elevations in AST occurred in 76% (93/122) [see Adverse Reactions (6.1)]. Grade 3 ALT elevations were reported in 26% (32/122) of patients. Grade 3 AST elevations were reported in 30% (36/122) and Grade 4 AST elevations were reported in 3% (4/122) of patients. Elevated liver enzymes occurred in the majority of patients in Cycle 1 and were reversible following dose interruption.
Monitor alanine aminotransferase (ALT) and aspartate aminotransferase (AST) prior to each infusion with ELZONRIS. Withhold ELZONRIS temporarily if the transaminases rise to greater than 5 times the upper limit of normal and resume treatment upon normalization or when resolved [see Dosage and Administration (2.2)].
-
6 ADVERSE REACTIONS
The following serious adverse drug reactions are described elsewhere in the labeling:
- Capillary Leak Syndrome [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety of ELZONRIS was assessed in a single-arm clinical trial that included 122 adults with newly diagnosed or relapsed/refractory myeloid malignancies, including 86 with BPDCN, treated with ELZONRIS 12 mcg/kg daily for 5 days of a 21-day cycle. The overall median number of cycles started was 2.5 (range, 1-76), and 4 in patients with BPDCN (range, 1-76).
Four (3%) patients (4/122) had fatal adverse reactions, all of which were related to capillary leak syndrome. Overall, 8% (10/122) of patients discontinued treatment with ELZONRIS due to an adverse reaction; the most common adverse reactions resulting in treatment discontinuation were hepatic toxicities, hypoalbuminemia and CLS (2% each).
Table 3 summarizes the common (≥ 10%) adverse reactions with ELZONRIS in patients with myeloid malignancies. The rate of any given adverse reaction or lab abnormality was derived from all the reported events of that type.
Table 3. Adverse Reactions in ≥ 10% of Patients Receiving 12 mcg/kg of ELZONRIS 1 Capillary leak syndrome defined as any event reported as CLS during treatment with ELZONRIS or the occurrence of at least 2 of the following CLS manifestations within 7 days of each other: hypoalbuminemia (including albumin value less than 3.0 g/dL), edema (including weight increase of 5 kg or more), hypotension (including systolic blood pressure less than 90 mmHg).
N=122 All Grades
%Grade ≥ 3
%Vascular disorders Capillary leak syndrome1 53 11 Hypotension 25 7 Hypertension 14 6 General disorders and administration site conditions Fatigue 45 7 Pyrexia 43 0 Peripheral edema 39 1 Chills 26 1 Gastrointestinal disorders Nausea 45 0 Constipation 24 0 Diarrhea 21 0 Vomiting 19 0 Investigations Weight increase 31 0 Nervous system disorders Headache 28 0 Dizziness 21 0 Metabolism and nutrition disorders Decreased appetite 22 0 Respiratory, thoracic and mediastinal disorders Dyspnea 20 3 Epistaxis 12 1 Cough 12 0 Blood and lymphatic system disorders Febrile neutropenia 19 16 Musculoskeletal and connective tissue disorders Back pain 19 2 Pain in extremity 10 2 Cardiac disorders Tachycardia 17 0 Psychiatric disorders Insomnia 16 0 Anxiety 15 0 Skin and subcutaneous tissue disorders Pruritus 10 0 Clinically relevant adverse reactions occurring in less than 10% of patients treated with ELZONRIS included tumor lysis syndrome.
Table 4 summarizes the clinically important laboratory abnormalities that occurred in ≥ 10% patients with myeloid malignancies treated with ELZONRIS.
Table 4. Selected Laboratory Abnormalities in Patients Receiving 12 mcg/kg of ELZONRIS Treatment-Emergent
Laboratory AbnormalitiesAll Grades
%Grade ≥ 3
%Hematology Platelets decrease 68 49 Hemoglobin decrease 61 30 Neutrophils decrease 38 29 Chemistry Glucose increase 89 21 ALT increase 79 26 AST increase 76 33 Albumin decrease 72 1 Calcium decrease 57 2 Sodium decrease 52 9 Potassium decrease 36 6 Phosphate decrease 32 10 Creatinine increase 26 0 Magnesium decrease 25 0 Alkaline phosphatase increase 22 1 Potassium increase 20 3 Magnesium increase 13 4 Bilirubin increase 11 0 Glucose decrease 10 0 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, ELZONRIS has the potential for adverse effects on embryo-fetal development [see Clinical Pharmacology (12.1)]. There are no available data on ELZONRIS use in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Animal reproduction or developmental toxicity studies have not been conducted with tagraxofusp-erzs. Advise pregnant women of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4%, and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
No data are available regarding the presence of ELZONRIS in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children from ELZONRIS, breast feeding is not recommended during treatment and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
Based on its mechanism of action, ELZONRIS may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]
Pregnancy Testing:
Conduct pregnancy testing in females of reproductive potential within 7 days prior to initiating ELZONRIS treatment.
Contraception:
Advise females to use acceptable contraceptive methods during ELZONRIS treatment and for 1 week after the last dose of ELZONRIS.
8.4 Pediatric Use
The safety and effectiveness of ELZONRIS for treatment of BPDCN have been established in pediatric patients 2 years of age and older (no data for pediatric patients less than 2 years of age). Use of ELZONRIS in these age groups is supported by evidence from an adequate and well-controlled study of ELZONRIS in adults with BPDCN and additional safety data from three pediatric patients with BPDCN, including 1 child (2 years to < 12 years old) and 2 adolescents (12 years to < 17 years old), treated with ELZONRIS at the recommended dosage. The safety profile of ELZONRIS in the pediatric patients was similar to that seen in the adults. Efficacy for pediatric patients is extrapolated from the results of STML-401-0114 [see Clinical Studies 14.1, 14.2]. Safety and effectiveness have not been established in pediatric patients younger than 2 years of age.
8.5 Geriatric Use
Of the 86 patients who received ELZONRIS for BPDCN at the labeled dose in STML-401-0114, 63% were 65 years and older and 22% were 75 years and older. Patients 75 years or older experienced a higher incidence of altered mental status (including confusional state, delirium, mental status changes, dementia, and encephalopathy) than patients under 75 years of age.
-
11 DESCRIPTION
Tagraxofusp-erzs, a CD123-directed cytotoxin, is a fusion protein comprised of a recombinant human interleukin-3 (IL-3) and truncated diphtheria toxin (DT). Tagraxofusp-erzs has an approximate molecular weight of 57,695 Daltons. Tagraxofusp-erzs is constructed by recombinant DNA technology and produced in Escherichia coli cells.
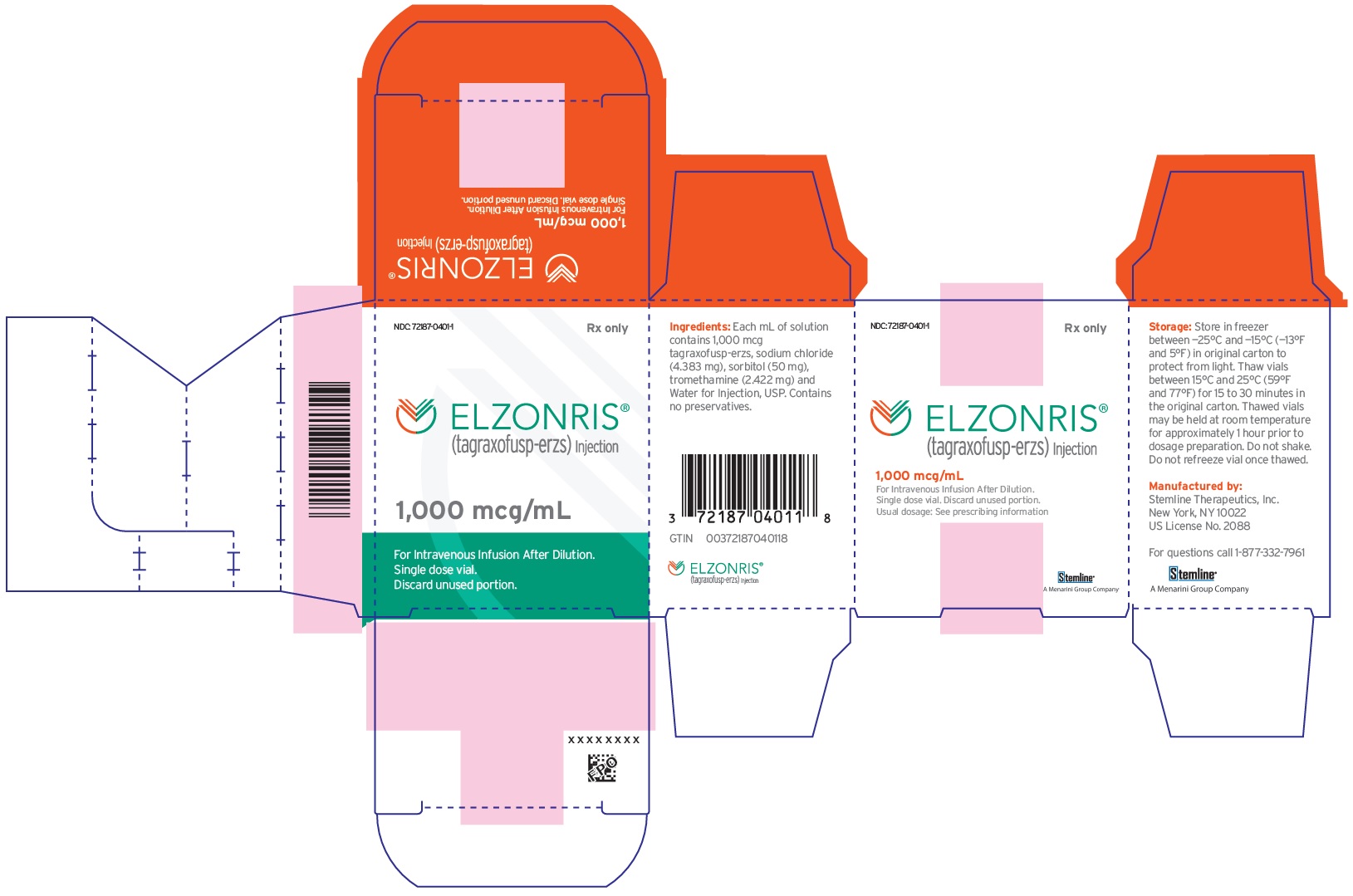
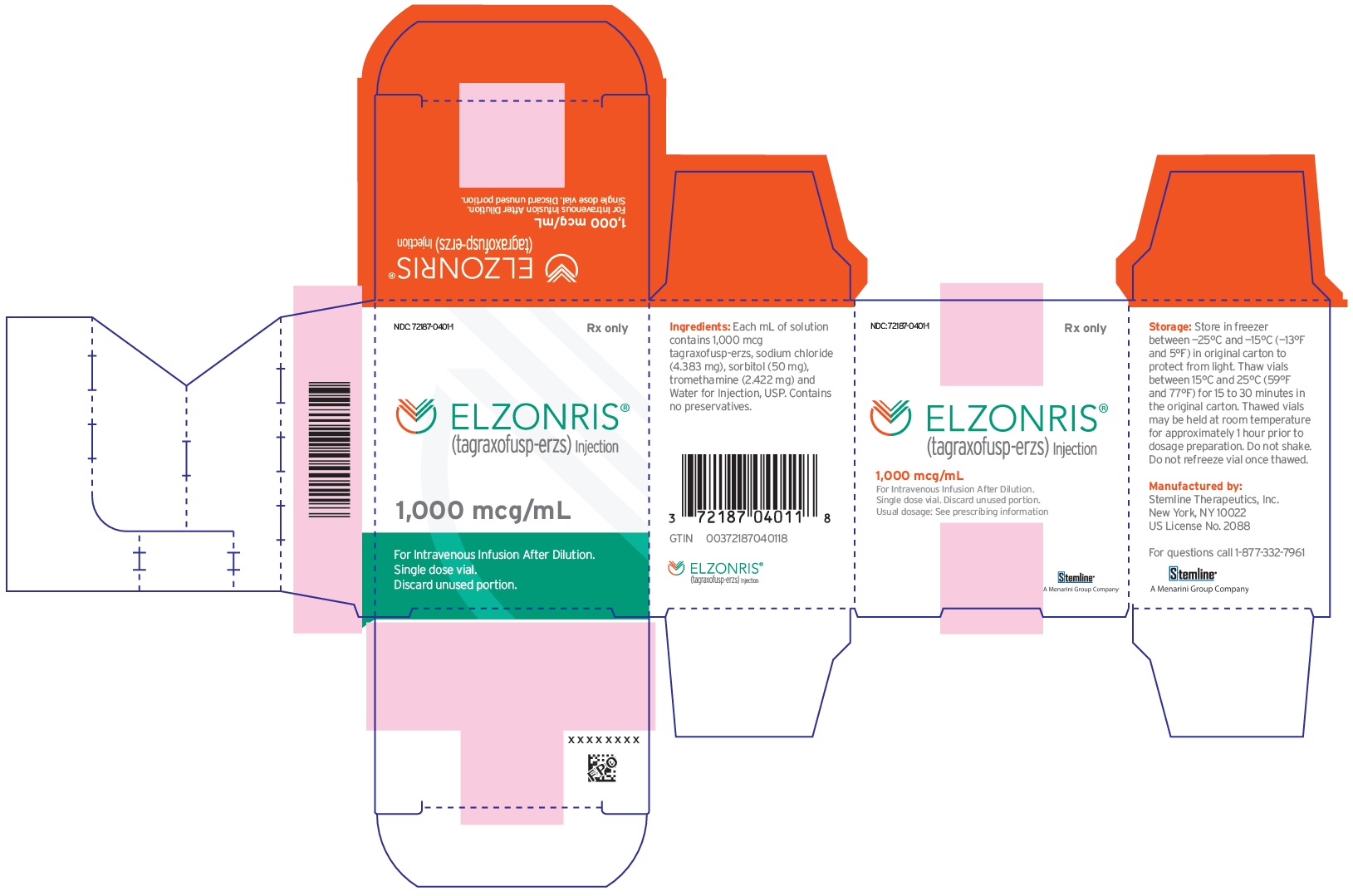
ELZONRIS (tagraxofusp-erzs) injection is a preservative-free, sterile, clear, colorless solution that may contain a few white to translucent particles and requires dilution prior to intravenous infusion. ELZONRIS is supplied at a concentration of 1,000 mcg/mL in a single-dose vial. Each mL of ELZONRIS contains 1,000 mcg tagraxofusp-erzs, sodium chloride (4.38 mg), sorbitol (50 mg), tromethamine (2.42 mg) and Water for Injection, USP and pH is 7.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tagraxofusp-erzs is a CD123-directed cytotoxin composed of recombinant human interleukin-3 (IL-3) and truncated diphtheria toxin (DT) fusion protein that inhibits protein synthesis and causes cell death in CD123-expressing cells.
12.2 Pharmacodynamics
The exposure-response relationships and the time course of pharmacodynamic response for ELZONRIS have not been fully characterized.
12.3 Pharmacokinetics
Following administration of tagraxofusp-erzs 12 mcg/kg via 15-minute infusion in patients with BPDCN, the mean (SD) area under the plasma drug concentration over time curve (AUC) was 231 (123) hr·mcg/L and maximum plasma concentration (Cmax) was 162 (58.1) mcg/L.
Distribution
Mean (SD) volume of distribution of tagraxofusp-erzs is 5.1 (1.9) L in patients with BPDCN.Elimination
Mean (SD) clearance is 7.1 (7.2) L/hr in patients with BPDCN. Mean (SD) terminal half-life of tagraxofusp-erzs is 0.7 (0.3) hours.Specific Population
No clinically significant differences in the pharmacokinetics of tagraxofusp-erzs were observed based on age (22 to 84 years), sex, mild to moderate renal impairment (eGFR 30 to 89 mL/min/1.73 m2, estimated by MDRD), mild (total bilirubin ≤ ULN and AST >ULN, or total bilirubin 1 to 1.5 times ULN and any AST) or moderate (total bilirubin >1.5 to 3 times ULN and any AST) hepatic impairment or body weight after adjusting dose by body weight. The effect of severe renal impairment (eGFR 15 to 29 mL/min/1.73 m2), or severe hepatic impairment (total bilirubin >3 times ULN and any AST) on tagraxofusp-erzs pharmacokinetics is unknown.Drug Interaction Studies
No drug-drug interaction studies have been conducted with ELZONRIS.12.6 Immunogenicity
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to ELZONRIS with the incidences of antibodies to other products may be misleading. Immune response to ELZONRIS was evaluated by assessment of serum binding reactivity against ELZONRIS (anti-drug antibodies; ADA) and neutralizing antibodies by inhibition of functional activity. Immune response to ELZONRIS was assessed using two immunoassays. The first assay detected reactivity directed against ELZONRIS (ADA), and the second assay detected reactivity against the interleukin-3 (IL-3) portion of ELZONRIS. Two cell-based assays were used to investigate the presence of neutralizing antibodies by inhibition of a cell-based functional activity.
In 130 patients treated with ELZONRIS in 4 clinical trials:
- 96% (115/120) of patients evaluable for the presence of pre-existing ADA at baseline before treatment were confirmed positive with 21% being positive for the presence of neutralizing antibodies. The high prevalence of ADA at baseline was anticipated due to diphtheria immunization.
- 99% (107/108) of patients evaluable for treatment-emergent ADA tested positive with most patients showing an increase in ADA titer by the end of Cycle 2 of ELZONRIS.
- 85% (86/101) of ADA-positive patients evaluable for the presence of neutralizing antibodies were neutralizing antibody-positive.
- 68% (73/108) of patients evaluable for treatment-emergent anti-IL-3 antibodies tested positive with most patients testing positive by Cycle 3 of ELZONRIS.
Anti-Product Antibody Effects on Pharmacokinetics
The presence of ADA had a clinically significant effect on the pharmacokinetics of tagraxofusp-erzs. Pharmacokinetic data obtained following doses given in Cycle 3 showed increased titers of anti-drug antibodies and reduced free ELZONRIS concentration in most plasma samples. Following administration of tagraxofusp-erzs 12 mcg/kg via 15-minute infusion in patients with pre-existing anti-drug antibodies, the mean (SD) volume of distribution of tagraxofusp-erzs is 21.2 (25.4) L, clearance is 13.9 (19.4) L/hr, AUC is 151 (89.2) hr·mcg/L and Cmax is 80.0 (82.2) mcg/L. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to assess the carcinogenic or genotoxic potential of tagraxofusp. Animal fertility studies have not been conducted with tagraxofusp-erzs.
13.2 Animal Toxicology and/or Pharmacology
At human equivalent doses greater than or equal to 1.6 times the recommended dose based on body surface area, severe kidney tubular degeneration/necrosis was observed in cynomolgus monkeys. At human equivalent doses equal to the recommended dose, degeneration/necrosis of the choroid plexus in the brain was observed in cynomolgus monkeys. The reversibility of this finding was not assessed at lower doses, but the finding was irreversible and became progressively more severe at a human equivalent dose 1.6 times the recommended dose, 3 weeks after dosing stopped.
-
14 CLINICAL STUDIES
14.1 First-Line Treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
STML-401-0114 (NCT 02113982; Study 0114) was a multicenter, open-label, single-arm, clinical trial that included a prospective cohort of 13 patients with treatment-naive BPDCN. Treatment consisted of ELZONRIS 12 mcg/kg intravenously over 15 minutes once daily on days 1 to 5 of a 21-day cycle. Patient baseline characteristics are presented in Table 5.
Table 5. Baseline Demographics of Patients with Treatment-Naive BPDCN Parameter N=13 Gender, N (%) Male 11 (84.6) Female 2 (15.4) Age (years), N (%) Median 65.0 Minimum, Maximum 22, 84 ECOG, N (%) 0 8 (61.5) 1 5 (38.5) BPDCN at Baseline, N (%) Skin 13 (100.0) Bone Marrow 7 (53.8) Peripheral Blood 3 (23.1) Lymph Nodes 6 (46.2) Viscera 2 (15.4) The efficacy of ELZONRIS in patients with treatment-naive BPDCN was based on the rate of complete response or clinical complete response (CR/CRc). Key efficacy measures are presented in Table 6. The median time to CR/CRc was 57 days (range: 14 to 107).
Table 6. Efficacy Measures in Patients with Treatment-Naive BPDCN * CRc is defined as complete response with residual skin abnormality not indicative of active disease.
Parameter N=13 CR/CRc* Rate, N (%) 7 (53.8) (95% CI) (25.1, 80.8) Duration of CR/CRc (months) Median Not reached Minimum, Maximum 3.9, 12.2 Duration of follow up (months) Median 11.5 Minimum, Maximum 0.2, 12.7 14.2 Relapsed or Refractory Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
STML-401-0114 (NCT02113982; Study 0114) was a multicenter, open-label, single-arm, clinical trial that included 15 patients with relapsed or refractory BPDCN. Treatment consisted of ELZONRIS 12 mcg/kg on days 1 to 5 of each 21-day cycle. Patient baseline characteristics are presented in Table 7.
Table 7. Baseline Demographics of Patients with Relapsed or Refractory BPDCN Parameter N=15 Gender, N (%) Male 13 (86.7) Female 2 (13.3) Age (years) Median 72 Minimum, Maximum 44, 80 ECOG, N (%) 0 5 (33.3) 1 10 (66.7) BPDCN at Baseline, N (%) Skin 13 (86.7) Bone marrow 9 (60.0) Lymph node 8 (53.3) Visceral 4 (26.7) Peripheral blood 1 (6.7) In the 15 patients with relapsed/refractory BPDCN, one patient achieved a CR (duration: 111 days) and one patient achieved a CRc (duration: 424 days).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ELZONRIS (tagraxofusp-erzs) injection is a preservative-free, sterile, clear, colorless, 1,000 mcg in 1 mL solution supplied in a single-dose glass vial. Each carton contains one vial (NDC 72187-0401-1).
Storage and Handling
Store in freezer between -25°C and -15°C (-13°F and 5°F). Protect ELZONRIS from light by storing in the original package until time of use. Thaw vials at room temperature between 15°C and 25°C (59°F and 77°F) prior to preparation [see Dosage and Administration (2.3)]. Do not refreeze the vial once thawed. Do not use beyond expiration date on container.
-
17 PATIENT COUNSELING INFORMATION
Capillary Leak Syndrome
Advise patients of the risk of capillary leak syndrome (CLS), and to contact their health care professional for signs and symptoms associated with CLS including new or worsening edema, weight gain, shortness of breath, and/or hypotension after infusion. Advise patients to weigh themselves daily [see Warnings and Precautions (5.1)].
Hypersensitivity
Advise patients of the risk of hypersensitivity reactions, and to contact their healthcare professional for signs and symptoms associated with hypersensitivity reactions including rash, flushing, wheezing and swelling of the face [see Warnings and Precautions (5.2)].
Hepatotoxicity
Advise patients to report symptoms that may indicate elevated liver enzymes including fatigue, anorexia and/or right upper abdominal discomfort [see Warnings and Precautions (5.3)].
Contraception
Advise females to avoid pregnancy and to use acceptable contraceptive methods during ELZONRIS treatment and for 1 week after the last dose of ELZONRIS [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed [see Use in Specific Populations (8.2)].
Manufactured by:
Stemline Therapeutics, Inc.
New York, NY 10022
US License No. 2088
Stemline®
A Menarini Group Company - PRINCIPAL DISPLAY PANEL - NDC: 72187-0401-1 - 1000 mcg/mL Vial Label
- PRINCIPAL DISPLAY PANEL - NDC: 72187-0401-1 - 1000 mcg/mL Carton Label
-
INGREDIENTS AND APPEARANCE
ELZONRIS
tagraxofusp injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72187-0401 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAGRAXOFUSP (UNII: 8ZHS5657EH) (TAGRAXOFUSP - UNII:8ZHS5657EH) TAGRAXOFUSP 1000 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 4.38 mg in 1 mL SORBITOL (UNII: 506T60A25R) 50 mg in 1 mL TROMETHAMINE (UNII: 023C2WHX2V) 2.42 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72187-0401-1 1 in 1 CARTON 01/18/2019 1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761116 01/18/2019 Labeler - Stemline Therapeutics, Inc. (139606136) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 832394733 MANUFACTURE(72187-0401)