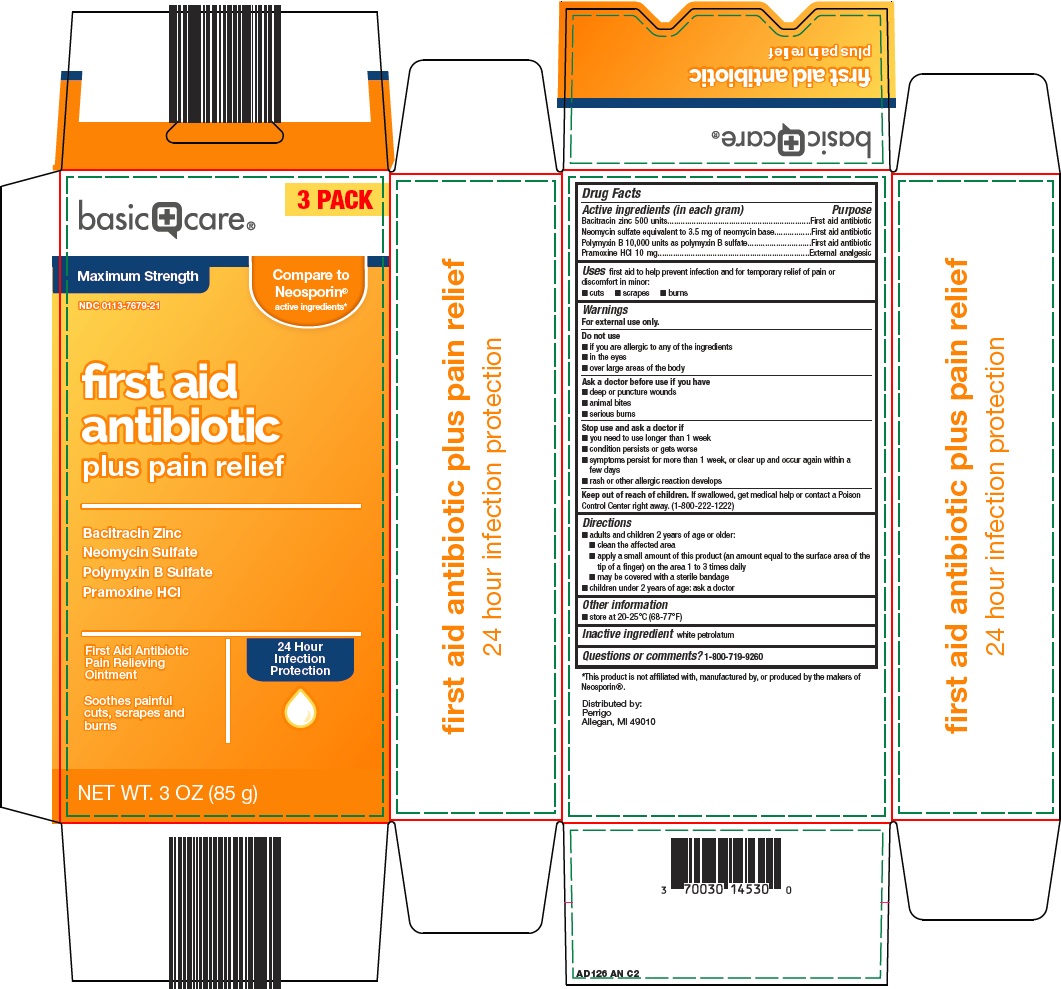
BASIC CARE FIRST AID ANTIBIOTIC PLUS PAIN RELIEF- bacitracin, neomycin, polymyxin b, pramoxine hcl ointment
L. Perrigo Company
----------
Amazon First Aid Antibiotic Plus Pain Relief Drug Facts
Active ingredient (in each gram)
Bacitracin zinc 500 units
Neomycin sulfate equivalent to 3.5 mg of neomycin base
Polymyxin B 10,000 units as polymyxin B sulfate
Pramoxine HCl 10 mg
Uses
first aid to help prevent infection and for temporary relief of pain or discomfort in minor:
- •
- cuts
- •
- scrapes
- •
- burns
Warnings
For external use only.
Do not use
- •
- if you are allergic to any of the ingredients
- •
- in the eyes
- •
- over large areas of the body
Directions
- •
- adults and children 2 years of age or older:
- •
- clean the affected area
- •
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- •
- may be covered with a sterile bandage
- •
- children under 2 years of age: ask a doctor
Principal Display Panel
3 PACK
Maximum Strength
Compare to Neosporin® active ingredients
first aid antibiotic plus pain relief
Bacitracin Zinc
Neomycin Sulfate
Polymyxin B Sulfate
Pramoxine HCl
First Aid Antibiotic
Pain Relieving
Ointment
24 Hour
Infection
Protection
Soothes painful cutes, scrapes and burns
NET WT. 3 OZ (85 g)
| BASIC CARE FIRST AID ANTIBIOTIC PLUS PAIN RELIEF
bacitracin, neomycin, polymyxin b, pramoxine hcl ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - L. Perrigo Company (006013346) |
Revised: 1/2024
Document Id: 639a4329-3c3b-439c-a889-a4f53e568ea6
Set id: 7fef39aa-cd70-4075-8f1d-6720b9e240e7
Version: 2
Effective Time: 20240114
L. Perrigo Company