PROSTIGMIN- neostigmine bromide tablet
Valeant Pharmaceuticals North America LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Prostigmin® (neostigmine bromide) Tablets
DESCRIPTION
Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15-mg tablets. Each tablet also contains gelatin, lactose, corn starch, stearic acid, sugar and talc.
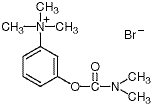
Chemically, neostigmine bromide is (m-hydroxy-phenyl)trimethylammonium bromide dimethylcarbamate. It is a white, crystalline, bitter powder, soluble 1:1 in water, with a molecular weight of 303.20 and the following structural formula:
CLINICAL PHARMACOLOGY
Neostigmine inhibits the hydrolysis of acetylcholine by competing with acetylcholine for attachment to acetylcholinesterase at sites of cholinergic transmission. It enhances cholinergic action by facilitating the transmission of impulses across neuromuscular junctions. It also has a direct cholinomimetic effect on skeletal muscle and possibly on autonomic ganglion cells and neurons of the central nervous system. Neostigmine undergoes hydrolysis by cholinesterase and is also metabolized by microsomal enzymes in the liver. Protein binding to human serum albumin ranges from 15 to 25 percent.
Neostigmine bromide is poorly absorbed from the gastrointestinal tract following oral administration. As a rule, 15 mg of neostigmine bromide orally is equivalent to 0.5 mg of neostigmine methylsulfate parenterally, due to poor absorption of the tablet from the intestinal tract. In a study in fasting myasthenic patients, the extent of absorption was estimated to be 1 to 2 percent of the ingested 30-mg single oral dose. Peak concentrations in plasma occurred 1 to 2 hours following drug ingestion, with considerable individual variations. The half-life ranged from 42 to 60 minutes with a mean half-life of 52 minutes.
INDICATIONS AND USAGE
Prostigmin is indicated for the symptomatic treatment of myasthenia gravis. Its greatest usefulness is in prolonged therapy where no difficulty in swallowing is present. In acture myasthenic crisis where difficulty in breathing and swallowing is present, the parenteral form (neostigmine methylsulfate) should be used. The patient can be transferred to the oral form as soon as it can be tolerated.
CONTRAINDICATIONS
Prostigmin is contraindicated in patients with known hypersensitivity to the drug. Because of the presence of the bromide ion, it should not be used in patients with a previous history of reaction to bromides. It is contraindicated in patients with peritonitis or mechanical obstruction of the intestinal or urinary tract.
WARNINGS
Prostigmin should be used with caution in patients with epilepsy, bronchial asthma, bradycardia, recent coronary occlusion, vagotonia, hyperthyroidism, cardiac arrhythmias or peptic ulcer. As a rule, 15 mg of neostigmine bromide orally is equivalent to 0.5 mg of neostigmine methylsulfate parenterally, due to poor absorption of the tablet from the intestinal tract. Large doses should be avoided in situations where there might be an increased absorption rate from the intestinal tract. It should be used with caution when co-administered with anticholinergic drugs, in order to avoid reduction in intestinal mobility.
PRECAUTIONS
General:
It is important to differentiate between myasthenic crisis and cholinergic crisis caused by overdosage of Prostigmin. Both conditions result in extreme muscle weakness but require radically different treatment. (See OVERDOSAGE section.)
Drug Interactions:
Certain antibiotics, especially neomycin, streptomycin and kanamycin, have a mild but definite nondepolarizing blocking action which may accentuate neuromuscular block. These antibiotics should be used in the myasthenic patient only where definitely indicated, and then careful adjustment should be made of adjunctive anticholinesterase dosage.
Local and some general anesthetics, antiarrhythmic agents and other drugs that interfere with neuromuscular transmission should be used cautiously, if at all, in patients with myasthenia gravis; the dose of Prostigmin may have to be increased accordingly.
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
There have been no studies with Prostigmin which would permit an evaluation of its carcinogenic or mutagenic potential. Studies on the effect of Prostigmin on fertility and reproduction have not been performed.
Pregnancy:
Teratogenic Effects: Pregnancy Category C
There are no adequate or well-controlled studies of Prostigmin in either laboratory animals or in pregnant women. It is not known whether Prostigmin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Prostigmin should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
It is not known whether Prostigmin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from Prostigmin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
Side effects are generally due to an exaggeration of pharmacological effects of which salivation and fasciculation are the most common. Bowel cramps and diarrhea may also occur.
The following additional adverse reacions have been reported following the use of either neostigmine bromide or neostigmine methylsulfate:
Allergic: Allergic reactions and anaphylaxis.
Neurologic: Dizziness, convulsions, loss of consciousness, drowsiness, headache, dysarthria, miosis and visual changes.
Cardiovascular: Cardiac arrhythmias (including bradycardia, tachycardia, A-V block and nodal rhythm) and nonspecific EKG changes have been reported, as well as cardiac arrest, syncope and hypotension. These have been predominantly noted following the use of the injectable form of Prostigmin.
Respiratory: Increased oral, pharyngeal and brochial secretions, and dyspnea. Respiratory depression, respiratory arrest and bronchospasm have been reported following the use of the injectable form of Prostigmin.
Dermatologic: Rash and urticaria.
Gastrointestinal: Nausea, emesis, flatulence and increased peristalsis.
Genitourinary: Urinary frequency.
Musculoskeletal: Muscle cramps and spasms, arthralgia.
Miscellaneous: Diaphoresis, flushing and weakness.
OVERDOSAGE
Overdosage of Prostigmin can cause cholinergic crisis, which is characterized by increasing muscle weakness, and through involvement of the muscles of respiration, may result in death. Myasthenic crisis, due to an increase in the severity of the disease, is also accompanied by extreme muscle weakness and may be difficult to distinguish from cholinergic crisis on a symptomatic basis. However, such differentiation is extremely important because increases in the dose of Prostigmin or other drugs in this class, in the presence of cholinergic crisis or of a refractory or "insensitive" state, could have grave consequences. The two types of crises may be differentiated by the use of Tensilon® (edrophonium chloride) as well as by clinical judgment.
Treatment of the two conditions differs radically. Whereas the presence of myasthenic crisis requires more intensive anticholinesterase therapy, cholinergic crisis calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended.
Atropine may also be used to abolish or minimize gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis.
The LD50 of neostigmine methylsulfate in mice is 0.3±0.02 mg/kg intravenously, 0.54±0.03 mg/kg subcutaneously, and 0.395±0.025 mg/kg intramuscularly; in rats the LD50 is 0.315±0.019 mg/kg intravenously, 0.445±0.032 mg/kg subcutaneously, and 0.423±0.032 mg/kg intramuscularly.
DOSAGE AND ADMINISTRATION
The onset of action of Prostigmin given orally is slower than when given parenterally, but the duration of action is longer and the intensity of action more uniform. Dosage requirements for optimal results vary from 15 mg to 375 mg per day. In some instances it may be necessary to exceed these dosages, but the possibility of cholinergic crisis must be recognized. The average dose is 10 tablets (150 mg) administered over a 24-hour period. The interval between doses is of paramount importance. The dosage schedule should be adjusted for each patient and changed as the need arises. Frequently, therapy is required day and night. Larger portions of the total daily dose may be given at times when the patient is more prone to fatigue (afternoon, mealtimes, etc.). The patient should be encouraged to keep a daily record of his or her condition to assist the physician in determining an optimal therapeutic regimen.
HOW SUPPLIED
Scored, white tablets containing 15 mg neostigmine bromide—bottles of 100 (NDC 0187-3100-10). Imprint on tablets: (front) PROSTIGMIN 15; (back) V.
| PROSTIGMIN
neostigmine bromide tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Valeant Pharmaceuticals North America LLC (042230623) |
